Benfotiamine: Difference between revisions
→Research: remove pile of primary sources and simplify |
→Research: add ref and content |
||
| Line 64: | Line 64: | ||
==Research== |
==Research== |
||
Benfotiamine has been studied in laboratory models of [[diabetic retinopathy]], [[neuropathy]], and [[nephropathy]].<ref name="pmid20188835">{{cite journal |vauthors=Balakumar P, Rohilla A, Krishan P, Solairaj P, Thangathirupathi A |title=The multifaceted therapeutic potential of benfotiamine |journal=Pharmacol Res |volume=61 |issue=6 |pages=482–8 |year=2010 |pmid=20188835 |doi=10.1016/j.phrs.2010.02.008}}</ref> |
Benfotiamine has been studied in laboratory models of [[diabetic retinopathy]], [[neuropathy]], and [[nephropathy]].<ref name="pmid20188835">{{cite journal |vauthors=Balakumar P, Rohilla A, Krishan P, Solairaj P, Thangathirupathi A |title=The multifaceted therapeutic potential of benfotiamine |journal=Pharmacol Res |volume=61 |issue=6 |pages=482–8 |year=2010 |pmid=20188835 |doi=10.1016/j.phrs.2010.02.008}}</ref> As of 2015 there had been one clinical study of benfotiamine in [[diabetic neuropathy].<ref>{{cite journal|last1=Raval|first1=AD|last2=Thakker|first2=D|last3=Rangoonwala|first3=AN|last4=Gor|first4=D|last5=Walia|first5=R|title=Vitamin B and its derivatives for diabetic kidney disease.|journal=The Cochrane database of systematic reviews|date=12 January 2015|volume=1|pages=CD009403|doi=10.1002/14651858.CD009403.pub2|pmid=25579852}}</ref> |
||
Administration of benfotiamine may increase intracellular levels of [[thiamine diphosphate]], a cofactor of [[transketolase]].<ref name="pmid20188835" /> |
Administration of benfotiamine may increase intracellular levels of [[thiamine diphosphate]], a cofactor of [[transketolase]].<ref name="pmid20188835" /> |
||
Revision as of 20:11, 14 March 2017
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Milgamma |
| Other names | S-Benzoylthiamine O-monophosphate |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.040.906 |
| Chemical and physical data | |
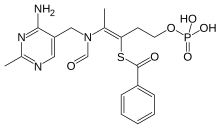
| Formula | C19H23N4O6PS |
| Molar mass | 466.448 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Benfotiamine (rINN, or S-benzoylthiamine O-monophosphate) is a synthetic S-acyl derivative of thiamine (vitamin B1).
It has been licensed for use in Germany since 1993 under the trade name Milgamma. (Combinations with pyridoxine or cyanocobalamin are also sold under this name.) It is prescribed there for treating sciatica and other painful nerve conditions.
It is marketed as a medicine and/or dietary supplement, depending on the respective Regulatory Authority.[citation needed]
Uses
Benfotiamine is primarily marketed as an antioxidant dietary supplement.
Pharmacology
After absorption, benfotiamine can be dephosphorylated by cells bearing an ecto-alkaline phosphatase to the lipid-soluble S-benzoylthiamine.[1] Benfotiamine should not be confused with allithiamine, a naturally occurring thiamine disulfide derivative with a distinct pharmacological profile.[2]
Research
Benfotiamine has been studied in laboratory models of diabetic retinopathy, neuropathy, and nephropathy.[3] As of 2015 there had been one clinical study of benfotiamine in [[diabetic neuropathy].[4]
Administration of benfotiamine may increase intracellular levels of thiamine diphosphate, a cofactor of transketolase.[3]
See also
References
- ^ Yamazaki, M (1968). "Studies on the absorption of S-benzoylthiamine O-monophosphate : (I) Metabolism in tissue homogenates". Vitamins. 38 (1): 12–20.
- ^ Volvert, M.L.; Seyen, S.; Piette, M.; Evrard, B.; Gangolf, M.; Plumier, J.C.; Bettendorff, L. (2008). "Benfotiamine, a synthetic S-acyl thiamine derivative, has different mechanisms of action and a different pharmacological profile than lipid-soluble thiamine disulfide derivatives". BMC Pharmacology. 8 (1): 10. doi:10.1186/1471-2210-8-10. PMC 2435522. PMID 18549472.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Balakumar P, Rohilla A, Krishan P, Solairaj P, Thangathirupathi A (2010). "The multifaceted therapeutic potential of benfotiamine". Pharmacol Res. 61 (6): 482–8. doi:10.1016/j.phrs.2010.02.008. PMID 20188835.
- ^ Raval, AD; Thakker, D; Rangoonwala, AN; Gor, D; Walia, R (12 January 2015). "Vitamin B and its derivatives for diabetic kidney disease". The Cochrane database of systematic reviews. 1: CD009403. doi:10.1002/14651858.CD009403.pub2. PMID 25579852.
