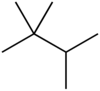
Triptane
(Redirected from 2,2,3-Trimethylbutane)
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2,2,3-Trimethylbutane[1] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1730756 | |||
| ChemSpider | |||
| ECHA InfoCard | 100.006.680 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| UN number | 1206 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C7H16 | |||
| Molar mass | 100.205 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Odorless | ||
| Density | 0.693 g mL−1 | ||
| Melting point | −26 to −24 °C; −15 to −11 °F; 247 to 249 K | ||
| Boiling point | 80.8 to 81.2 °C; 177.3 to 178.1 °F; 353.9 to 354.3 K | ||
| Vapor pressure | 23.2286 kPa (at 37.7 °C) | ||
Henry's law
constant (kH) |
4.1 nmol Pa−1 kg−1 | ||
| -88.36·10−6 cm3/mol | |||
Refractive index (nD)
|
1.389 | ||
| Thermochemistry | |||
Heat capacity (C)
|
213.51 J K−1 mol−1 | ||
Std molar
entropy (S⦵298) |
292.25 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−238.0 – −235.8 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−4.80449 – −4.80349 MJ mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
   
| |||
| Danger | |||
| H225, H302, H305, H315, H336, H400 | |||
| P210, P261, P273, P301+P310, P331 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −7 °C (19 °F; 266 K) | ||
| 450 °C (842 °F; 723 K) | |||
| Explosive limits | 1–7% | ||
| Related compounds | |||
Related alkanes
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Triptane, or 2,2,3-trimethylbutane, is an organic chemical compound with the molecular formula C7H16 or (H3C-)3C-C(-CH3)2H. It is therefore an alkane, specifically the most compact and heavily branched of the heptane isomers, the only one with a butane (C4) backbone.
Triptane is commonly used as an anti-knock additive in aviation fuels.
See also[edit]
References[edit]
- ^ "Triptan - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 11 March 2012.