Arsphenamine
Arsphenamine, also known as Salvarsan and 606, is a drug that was used beginning in the 1910s to treat syphilis and trypanosomiasis. It is an organoarsenic compound and was the first modern chemotherapeutic agent.
History
Sahachiro Hata discovered the anti-syphilitic activity of this compound in 1909 in the laboratory of Paul Ehrlich, during a survey of hundreds of newly-synthesized organic arsenical compounds. Paul Ehrlich had theorized that, by screening many compounds, a drug could be discovered with anti-microbial activity. Ehrlich's team began their search for such a magic bullet among chemical derivatives of the dangerously toxic drug atoxyl. This was the first organized team effort to optimize the biological activity of a lead compound through systematic chemical modifications, the basis for nearly all modern pharmaceutical research.
Arsphenamine was originally called 606 because it was the 6th in the 6th group of compounds synthesized for testing; it was marketed by Hoechst AG under the trade name Salvarsan in 1910.[1][2] Salvarsan was the first organic anti-syphillitic, and a great improvement over the inorganic mercury compounds that had been used previously. It was distributed as a yellow, crystalline, hygroscopic powder that was highly unstable in air. [3] This significantly complicated administration, as the drug needed to be dissolved in several hundred milliliters of distilled, sterile water using a means which minimized its exposure to air to create a solution suitable for injection. Some of the side effects attributed to Salvarsan were actually caused by improper handling and administration, causing Ehrlich, who worked assiduously to standardize practices, to observe "the step from the laboratory to the patient's bedside ... is extraordinarily arduous and fraught with danger." [1]
Ehrlich's laboratory developed a more soluble (but slightly less effective) arsenical compound, Neosalvarsan, (neoarsphenamine), which was easier to prepare and became available in 1912. These arsenical compounds came with considerable risk of side effects, and they were supplanted as treatments for syphilis in the 1940s by penicillin.
After leaving Erlich's laboratory, Sahachiro Hata continued parallel investigation of the new medicine in Japan.[4]
Mechanism
The bacterium that causes syphilis is a spirochete, Treponema pallidum. Arsphenamine is not toxic to spirochetes until it has been converted to an active form by the body.
Structure
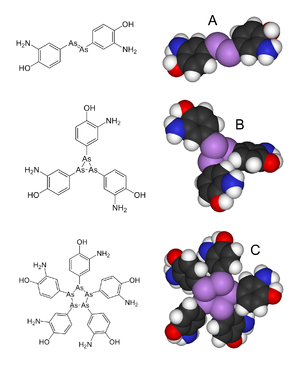
From Salvarsan's discovery until recently it was believed that the structure featured an As=As double bond. However, in 2005, an extensive mass spectral analysis showed that the actual structure is most likely to be a mixture of the cyclic trimer and a pentamer.[1][5] The revised structure features As-As single bonds, not double bonds.
References
- ^ a b c "Salvarsan". Chemical & Engineering News. Retrieved 2010-02-01.
- ^ In Germany it was the practice to designate compounds by their development number. Another compound known commonly in Germany by its number is Parathion, which was the 605th compound to be developed in search for insecticide. It is commonly known as E605 (E stands for Entwicklungsnummer (German for "development number")
- ^ "A Handbook of Useful Drugs". American Medical Association. 1913. Retrieved 2010-08-17.
- ^ Izumi, Yoshio; and Isozumi, Kazuo (2001). "Modern Japanese medical history and the European influence" (free download pdf). Keio Journal of Medicine. 50 (2): 91–99. PMID 11450598.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lloyd NC, Morgan HW, Nicholson BK, Ronimus RS (2005). "The composition of Ehrlich's salvarsan: resolution of a century-old debate". Angew. Chem. Int. Ed. Engl. 44 (6): 941–4. doi:10.1002/anie.200461471. PMID 15624113.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
