Clathrate compound
A clathrate is a chemical substance consisting of a lattice that traps or contains molecules. The word clathrate is derived from the Latin clathratus (clatratus), meaning ‘with bars, latticed’.[1] Most clathrate compounds are polymeric and completely envelop the guest molecule, but in modern usage clathrates also include host–guest complexes and inclusion compounds.[2] According to IUPAC, clathrates are inclusion compounds "in which the guest molecule is in a cage formed by the host molecule or by a lattice of host molecules."[3] The term refers to many molecular hosts, including calixarenes and cyclodextrins and even some inorganic polymers such as zeolites.
Clathrate hydrates are derived from organic hydrogen-bonded frameworks. These frameworks are prepared from molecules that "self-associate" by multiple hydrogen-bonding interactions.
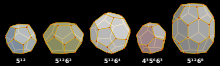
Most common clathrate crystal structures can be composed of cavities such as dodecahedral, tetrakaidecahedral and hexakaidecahedral cavities.

Examples

- Clathrates are formed when hydroquinone (PQ) solutions crystallize under pressure of some noble gases (G). They have the formula (PQ)3G. Crystals can be handled at room temperature but the noble gas is released upon dissolving the crystals.[6]
- Methane clathrates feature the hydrogen-bonded framework contributed by water and the guest molecules of methane. Large amounts of methane naturally frozen in this form exist both in permafrost formations and under the ocean sea-bed.[7] Other hydrogen-bonded networks are derived from hydroquinone, urea, and thiourea. A much studied host molecule is Dianin's compound.

- Hofmann clathrates are coordination polymers with the formula Ni(CN)4·Ni(NH3)2(arene). These materials crystallize with small aromatic guests (benzene, certain xylenes), and this selectivity has been exploited commercially for the separation of these hydrocarbons.[2] Metal organic frameworks (MOFs) form clathrates.

History
Clathrate hydrates were discovered in 1810 by Humphry Davy.[8] Clathrates were studied by P. Pfeiffer in 1927 and in 1930, E. Hertel defined "molecular compounds" as substances decomposed into individual components following the mass action law in solution or gas state. In 1945, H. M. Powell analyzed the crystal structure of these compounds and named them clathrates.
Related materials
Inclusion compounds are often molecules, whereas clathrates are typically polymeric[citation needed]. Intercalation compounds are not 3-dimensional, unlike clathrate compounds. Photolytically-sensitive caged compounds have been examined as containers for releasing a drug or reagent.[9]
See also
References
- ^ Latin dictionary Archived 2012-04-14 at the Wayback Machine
- ^ a b Atwood, J. L. (2012) "Inclusion Compounds" in Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim. doi:10.1002/14356007.a14_119
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "clathrates". doi:10.1351/goldbook.C01097
- ^ a b Krishna, Lakshmi; Koh, Carolyn A. (February 2015). "Inorganic and methane clathrates: Versatility of guest–host compounds for energy harvesting". MRS Energy & Sustainability. 2 (1): 8. doi:10.1557/mre.2015.9. ISSN 2329-2229.
- ^ Birchall, T.; Frampton, C. S.; Schrobilgen, G. J.; Valsdóttir, J. (1989). "Β-Hydroquinone xenon clathrate". Acta Crystallographica Section C Crystal Structure Communications. 45 (6): 944–946. doi:10.1107/S0108270188014556.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 893. ISBN 978-0-08-037941-8.
- ^ Pearce, Fred (27 June 2009). "Ice on fire: The next fossil fuel". New Scientist. No. 2714. pp. 30–33. Archived from the original on April 13, 2016. Retrieved July 5, 2009.
- ^ Thomas, Ellen (November 2004). "Clathrates: little known components of the global carbon cycle". Wesleyan University. Retrieved 13 December 2007.
- ^ Ellis-Davies, Graham C. R. (2007). "Caged compounds: Photorelease technology for control of cellular chemistry and physiology". Nature Methods. 4 (8): 619–628. doi:10.1038/nmeth1072. PMC 4207253. PMID 17664946.
