Colorectal cancer
| Colorectal cancer | |
|---|---|
| Other names | Colon cancer, rectal cancer, bowel cancer |
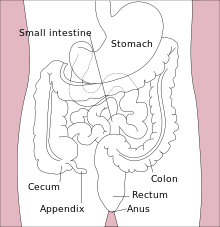 | |
| Diagram of the lower gastrointestinal tract | |
| Specialty | Oncology |
| Symptoms | Blood in the stool, change in bowel movements, weight loss, feeling tired all the time[1] |
| Causes | Old age, lifestyle factors, genetic disorders[2][3] |
| Risk factors | Diet, obesity, smoking, lack of physical activity, alcohol use[2][4] |
| Diagnostic method | Tissue biopsy during a sigmoidoscopy or colonoscopy[1] |
| Prevention | Screening from age of 50 to 75[5] |
| Treatment | Surgery, radiation therapy, chemotherapy, targeted therapy[6] |
| Prognosis | Five year survival rates 65% (USA)[7] |
| Frequency | 9.4 million (2015)[8] |
| Deaths | 832,000 (2015)[9] |
Colorectal cancer (CRC), also known as bowel cancer and colon cancer, is the development of cancer from the colon or rectum (parts of the large intestine).[6] A cancer is the abnormal growth of cells that have the ability to invade or spread to other parts of the body.[10] Signs and symptoms may include blood in the stool, a change in bowel movements, weight loss, and feeling tired all the time.[1]
Most colorectal cancers are due to old age and lifestyle factors with only a small number of cases due to underlying genetic disorders.[2][3] Some risk factors include diet, obesity, smoking, and lack of physical activity.[2][3] Dietary factors that increase the risk include red and processed meat as well as alcohol.[2][4] Another risk factor is inflammatory bowel disease, which includes Crohn's disease and ulcerative colitis.[2] Some of the inherited genetic disorders that can cause colorectal cancer include familial adenomatous polyposis and hereditary non-polyposis colon cancer; however, these represent less than 5% of cases.[2][3] It typically starts as a benign tumor, often in the form of a polyp, which over time becomes cancerous.[2]
Bowel cancer may be diagnosed by obtaining a sample of the colon during a sigmoidoscopy or colonoscopy.[1] This is then followed by medical imaging to determine if the disease has spread.[6] Screening is effective for preventing and decreasing deaths from colorectal cancer.[5] Screening, by one of a number of methods, is recommended starting from the age of 50 to 75.[5] During colonoscopy, small polyps may be removed if found.[2] If a large polyp or tumor is found, a biopsy may be performed to check if it is cancerous. Aspirin and other non-steroidal anti-inflammatory drugs decrease the risk.[2][11] Their general use is not recommended for this purpose, however, due to side effects.[12]
Treatments used for colorectal cancer may include some combination of surgery, radiation therapy, chemotherapy and targeted therapy.[6] Cancers that are confined within the wall of the colon may be curable with surgery while cancer that has spread widely are usually not curable, with management being directed towards improving quality of life and symptoms.[6] Five year survival rates in the United States are around 65%.[7] This, however, depends on how advanced the cancer is, whether or not all the cancer can be removed with surgery, and the person's overall health.[1] Globally, colorectal cancer is the third most common type of cancer, making up about 10% of all cases.[13] In 2012, there were 1.4 million new cases and 694,000 deaths from the disease.[13] It is more common in developed countries, where more than 65% of cases are found.[2] It is less common in women than men.[2]
Signs and symptoms

The signs and symptoms of colorectal cancer depend on the location of the tumor in the bowel, and whether it has spread elsewhere in the body (metastasis). The classic warning signs include: worsening constipation, blood in the stool, decrease in stool caliber (thickness), loss of appetite, loss of weight, and nausea or vomiting in someone over 50 years old.[14] While rectal bleeding or anemia are high-risk features in those over the age of 50,[15] other commonly described symptoms including weight loss and change in bowel habit are typically only concerning if associated with bleeding.[15][16]
Cause
Greater than 75–95% of colorectal cancer occurs in people with little or no genetic risk.[17][18] Risk factors include older age, male gender,[18] high intake of fat, alcohol, red meat, processed meats, obesity, smoking, and a lack of physical exercise.[17][19] Approximately 10% of cases are linked to insufficient activity.[20] The risk from alcohol appears to increase at greater than one drink per day.[21] Drinking 5 glasses of water a day is linked to a decrease in the risk of colorectal cancer and adenomatous polyps.[22] Streptococcus gallolyticus is associated with colorectal cancer.[23] Some strains of Streptococcus bovis/Streptococcus equinus complex are consumed by millions of people daily and thus may be safe.[24] 25 to 80% of people with Streptococcus bovis/gallolyticus bacteremia have concomitant colorectal tumors.[25] Seroprevalence of Streptococcus bovis/gallolyticus is considered as a candidate practical marker for the early prediction of an underlying bowel lesion at high risk population.[25] It has been suggested that the presence of antibodies to Streptococcus bovis/gallolyticus antigens or the antigens themselves in the bloodstream may act as markers for the carcinogenesis in the colon.[25]
Inflammatory bowel disease
People with inflammatory bowel disease (ulcerative colitis and Crohn's disease) are at increased risk of colon cancer.[26][27] The risk increases the longer a person has the disease,[28] and the worse the severity of inflammation.[29] In these high risk groups, both prevention with aspirin and regular colonoscopies are recommended.[28] People with inflammatory bowel disease account for less than 2% of colon cancer cases yearly.[29] In those with Crohn's disease 2% get colorectal cancer after 10 years, 8% after 20 years, and 18% after 30 years.[29] In those with ulcerative colitis approximately 16% develop either a cancer precursor or cancer of the colon over 30 years.[29]
Genetics
Those with a family history in two or more first-degree relatives (such as a parent or sibling) have a two to threefold greater risk of disease and this group accounts for about 20% of all cases. A number of genetic syndromes are also associated with higher rates of colorectal cancer. The most common of these is hereditary nonpolyposis colorectal cancer (HNPCC or Lynch syndrome) which is present in about 3% of people with colorectal cancer.[18] Other syndromes that are strongly associated with colorectal cancer include Gardner syndrome,[30] and familial adenomatous polyposis (FAP). For people with these syndromes, cancer almost always occurs and makes up 1% of the cancer cases.[31] A total proctocolectomy may be recommended for people with FAP as a preventative measure due to the high risk of malignancy. Colectomy, removal of the colon, may not suffice as a preventative measure because of the high risk of rectal cancer if the rectum remains.[32]
Most deaths due to colon cancer are associated with metastatic disease. A gene that appears to contribute to the potential for metastatic disease, metastasis associated in colon cancer 1 (MACC1), has been isolated.[33] It is a transcriptional factor that influences the expression of hepatocyte growth factor. This gene is associated with the proliferation, invasion and scattering of colon cancer cells in cell culture, and tumor growth and metastasis in mice. MACC1 may be a potential target for cancer intervention, but this possibility needs to be confirmed with clinical studies.[34]
Epigenetic factors, such as abnormal DNA methylation of tumor suppressor promoters play a role in the development of colorectal cancer.[35]
Pathogenesis
Colorectal cancer is a disease originating from the epithelial cells lining the colon or rectum of the gastrointestinal tract, most frequently as a result of mutations in the Wnt signaling pathway that increase signaling activity. The mutations can be inherited or acquired, and most probably occur in the intestinal crypt stem cell.[36][37][38] The most commonly mutated gene in all colorectal cancer is the APC gene, which produces the APC protein. The APC protein prevents the accumulation of β-catenin protein. Without APC, β-catenin accumulates to high levels and translocates (moves) into the nucleus, binds to DNA, and activates the transcription of proto-oncogenes. These genes are normally important for stem cell renewal and differentiation, but when inappropriately expressed at high levels, they can cause cancer. While APC is mutated in most colon cancers, some cancers have increased β-catenin because of mutations in β-catenin (CTNNB1) that block its own breakdown, or have mutations in other genes with function similar to APC such as AXIN1, AXIN2, TCF7L2, or NKD1.[39]
Beyond the defects in the Wnt signaling pathway, other mutations must occur for the cell to become cancerous. The p53 protein, produced by the TP53 gene, normally monitors cell division and kills cells if they have Wnt pathway defects. Eventually, a cell line acquires a mutation in the TP53 gene and transforms the tissue from a benign epithelial tumor into an invasive epithelial cell cancer. Sometimes the gene encoding p53 is not mutated, but another protective protein named BAX is mutated instead.[39]
Other proteins responsible for programmed cell death that are commonly deactivated in colorectal cancers are TGF-β and DCC (Deleted in Colorectal Cancer). TGF-β has a deactivating mutation in at least half of colorectal cancers. Sometimes TGF-β is not deactivated, but a downstream protein named SMAD is deactivated.[39] DCC commonly has a deleted segment of a chromosome in colorectal cancer.[40]
Approximately 70% of all human genes are expressed in colorectal cancer, with just over 1% of having increased expression in colorectal cancer compared to other forms of cancer.[41] Some genes are oncogenes: they are overexpressed in colorectal cancer. For example, genes encoding the proteins KRAS, RAF, and PI3K, which normally stimulate the cell to divide in response to growth factors, can acquire mutations that result in over-activation of cell proliferation. The chronological order of mutations is sometimes important. If a previous APC mutation occurred, a primary KRAS mutation often progresses to cancer rather than a self-limiting hyperplastic or borderline lesion.[42] PTEN, a tumor suppressor, normally inhibits PI3K, but can sometimes become mutated and deactivated.[39]
Comprehensive, genome-scale analysis has revealed that colorectal carcinomas can be categorized into hypermutated and non-hypermutated tumor types.[43] In addition to the oncogenic and inactivating mutations described for the genes above, non-hypermutated samples also contain mutated CTNNB1, FAM123B, SOX9, ATM, and ARID1A. Progressing through a distinct set of genetic events, hypermutated tumors display mutated forms of ACVR2A, TGFBR2, MSH3, MSH6, SLC9A9, TCF7L2, and BRAF. The common theme among these genes, across both tumor types, is their involvement in WNT and TGF-β signaling pathways, which results in increased activity of MYC, a central player in colorectal cancer.[43]
Field defects
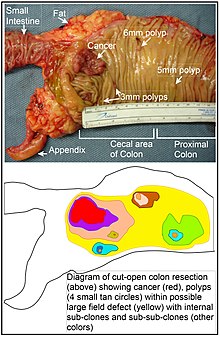
The term "field cancerization" was first used in 1953 to describe an area or "field" of epithelium that has been preconditioned (by what were largely unknown processes at the time) to predispose it towards development of cancer.[44] Since then, the terms "field cancerization", "field carcinogenesis", "field defect", and "field effect" have been used to describe pre-malignant or pre-neoplastic tissue in which new cancers are likely to arise.[45]
Field defects are important in progression to colon cancer.[46][47][48]
However, in most cancer research, as pointed out by Rubin[49] "The vast majority of studies in cancer research has been done on well-defined tumors in vivo, or on discrete neoplastic foci in vitro. Yet there is evidence that more than 80% of the somatic mutations found in mutator phenotype human colorectal tumors occur before the onset of terminal clonal expansion."[50] Similarly, Vogelstein et al.[51] pointed out that more than half of somatic mutations identified in tumors occurred in a pre-neoplastic phase (in a field defect), during growth of apparently normal cells. Likewise, epigenetic alterations present in tumors may have occurred in pre-neoplastic field defects.
An expanded view of field effect has been termed "etiologic field effect", which encompasses not only molecular and pathologic changes in pre-neoplastic cells but also influences of exogenous environmental factors and molecular changes in the local microenvironment on neoplastic evolution from tumor initiation to death.[52]
Epigenetics
Epigenetic alterations are much more frequent in colon cancer than genetic (mutational) alterations. As described by Vogelstein et al.,[51] an average cancer of the colon has only 1 or 2 oncogene mutations and 1 to 5 tumor suppressor mutations (together designated “driver mutations”), with about 60 further “passenger” mutations. The oncogenes and tumor suppressor genes are well studied and are described above under Pathogenesis.
However, by comparison, epigenetic alterations in colon cancers are frequent and affect hundreds of genes. For instance, there are types of small RNAs called microRNAs that are about 22 nucleotides long. These microRNAs (or miRNAs) do not code for proteins, but they can target protein coding genes and reduce their expression. Expression of these miRNAs can be epigenetically altered. As one example, the epigenetic alteration consisting of CpG island methylation of the DNA sequence encoding miR-137 reduces its expression. This is a frequent early epigenetic event in colorectal carcinogenesis, occurring in 81% of colon cancers and in 14% of the normal appearing colonic mucosa adjacent to the cancers. The altered adjacent tissues associated with these cancers are called field defects. Silencing of miR-137 can affect expression of about 500 genes, the targets of this miRNA.[53]
Changes in the level of miR-137 expression result in changed mRNA expression of the target genes by 2 to 20-fold and corresponding, though often smaller, changes in expression of the protein products of the genes. Other microRNAs, with likely comparable numbers of target genes, are even more frequently epigenetically altered in colonic field defects and in the colon cancers that arise from them. These include miR-124a, miR-34b/c and miR-342 which are silenced by CpG island methylation of their encoding DNA sequences in primary tumors at rates of 99%, 93% and 86%, respectively, and in the adjacent normal appearing mucosa at rates of 59%, 26% and 56%, respectively.[54][55]
In addition to epigenetic alteration of expression of miRNAs, other common types of epigenetic alterations in cancers that change gene expression levels include direct hypermethylation or hypomethylation of CpG islands of protein-encoding genes and alterations in histones and chromosomal architecture that influence gene expression.[56][57] As an example, 147 hypermethylations and 27 hypomethylations of protein coding genes were frequently associated with colorectal cancers. Of the hypermethylated genes, 10 were hypermethylated in 100% of colon cancers, and many others were hypermethylated in more than 50% of colon cancers.[58] In addition, 11 hypermethylations and 96 hypomethylations of miRNAs were also associated with colorectal cancers.[58]
Recent evidence indicates that early epigenetic reductions of DNA repair enzyme expression likely lead to the genomic and epigenomic instability characteristic of cancer.[46][59][60][61]
As summarized in the articles Carcinogenesis and Neoplasm, for sporadic cancers in general, a deficiency in DNA repair is occasionally due to a mutation in a DNA repair gene, but is much more frequently due to epigenetic alterations that reduce or silence expression of DNA repair genes.
Diagnosis
Colorectal cancer diagnosis is performed by sampling of areas of the colon suspicious for possible tumor development, typically during colonoscopy or sigmoidoscopy, depending on the location of the lesion.[18] It is confirmed by microscopical examination of a tissue sample.
Disease extent is usually determined by a CT scan of the chest, abdomen and pelvis.[18] Other potential imaging tests such as PET and MRI may be used in certain cases.[18]
Colon cancer staging is done next and is based on radiology and pathology. As for all other forms of cancer, tumor staging is based on the TNM system which considers how much the initial tumor has spread, if and where there are lymph node metastasis and if there are metastases in more distant organs, usually liver.[18]
The microscopic cellular characteristics of the tumor are reported from the analysis of tissue taken from a biopsy or surgery. A pathology report contains a description of the microscopical characteristics of the tumor tissue, including both tumor cells and how the tumor invades into healthy tissues and finally if the tumor appears to be completely removed. The most common form of colon cancer is adenocarcinoma (98% of cases).[62] Other, rarer types include lymphoma, adenosquamous and squamous cell carcinoma. Some subtypes have been found to be more aggressive.[63]
Macroscopy
Cancers on the right side of the large intestine (ascending colon and cecum) tend to be exophytic, that is, the tumor grows outwards from one location in the bowel wall. This very rarely causes obstruction of feces, and presents with symptoms such as anemia. Left-sided tumors tend to be circumferential, and can obstruct the bowel lumen, much like a napkin ring, and results in thinner caliber stools.
Microscopy
Adenocarcinoma is a malignant epithelial tumor, originating from superficial glandular epithelial cells lining the colon and rectum. It invades the wall, infiltrating the muscularis mucosae layer, the submucosa, and then the muscularis propria. Tumor cells describe irregular tubular structures, harboring pluristratification, multiple lumens, reduced stroma ("back to back" aspect). Sometimes, tumor cells are discohesive and secrete mucus, which invades the interstitium producing large pools of mucus. This occurs in mucinous adenocarcinoma, in which cells are poorly differentiated. If the mucus remains inside the tumor cell, it pushes the nucleus at the periphery, this occurs in "signet-ring cell." Depending on glandular architecture, cellular pleomorphism, and mucosecretion of the predominant pattern, adenocarcinoma may present three degrees of differentiation: well, moderately, and poorly differentiated.[64]
Immunochemistry
In cases where a metastasis from colorectal cancer is suspected, immunohistochemistry is used to ascertain correct diagnosis. Proteins that are more specifically expressed in colorectal cancer and can be used as diagnostic markers are cytokeratin 20, CDX2, SATB2 and CDH17. Most (50%) colorectal adenomas and (80–90%) colorectal cancer tumors are thought to over express the cyclooxygenase-2 (COX-2) enzyme.[65] This enzyme is generally not found in healthy colon tissue, but is thought to fuel abnormal cell growth.
Macroscopy
-
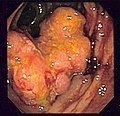
Appearance of the inside of the colon showing one invasive colorectal carcinoma (the crater-like, reddish, irregularly shaped tumor)
-
Gross appearance of a colectomy specimen containing two adenomatous polyps (the brownish oval tumors above the labels, attached to the normal beige lining by a stalk) and one invasive colorectal carcinoma (the crater-like, reddish, irregularly shaped tumor located above the label)
-
Endoscopic image of colon cancer identified in sigmoid colon on screening colonoscopy in the setting of Crohn's disease
-
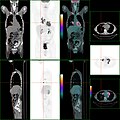
PET/CT of a staging exam of colon carcinoma. Besides the primary tumor a lot of lesions can be seen. On cursor position: lung nodule.
-
Fungating carcinoma of the colon
-
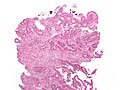
Cancer — Invasive adenocarcinoma (the most common type of colorectal cancer). The cancerous cells are seen in the center and at the bottom right of the image (blue). Near normal colon-lining cells are seen at the top right of the image.
-
Cancer — Histopathologic image of colonic carcinoid
-
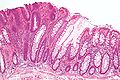
Precancer — Tubular adenoma (left of image), a type of colonic polyp and a precursor of colorectal cancer. Normal colorectal mucosa is seen on the right.
-
Precancer — Colorectal villous adenoma
Staging
Staging is typically made according to the TNM staging system from the WHO organization, the UICC and the AJCC. The Astler-Coller classification (1954) and the Dukes classification (1932) are now less used.
-
The T stages of bowel cancer.
-
Dukes stage A bowel cancer; the cancer is only in the inner lining of the bowel.
-
Dukes stage B bowel cancer; the cancer has invaded the muscle.
-
Dukes stage C bowel cancer; the cancer has invaded the nearby lymph nodes.
-
Dukes stage D bowel cancer; the cancer has metastasized.
The most common metastasis sites for colorectal cancer are the liver, the lung and the peritoneum.[66]
Tumor budding
Tumor budding in colorectal cancer is loosely defined by the presence of individual cells and small clusters of tumor cells at the invasive front of carcinomas. It has been postulated to represent an epithelial–mesenchymal transition (EMT). Tumor budding is a well-established independent marker of a potentially poor outcome in colorectal carcinoma that may allow for dividing people into risk categories more meaningful than those defined by TNM staging, and also potentially guide treatment decisions, especially in T1 and T3 N0 (Stage II, Dukes’ B) colorectal carcinoma. Unfortunately, its universal acceptance as a reportable factor has been held back by a lack of definitional uniformity with respect to both qualitative and quantitative aspects of tumor budding.[67]
Prevention
It has been estimated that about half of colorectal cancer cases are due to lifestyle factors and about a quarter of all cases are preventable.[68] Increasing surveillance, engaging in physical activity, consuming a diet high in fiber, and reducing smoking and alcohol consumption decrease the risk.[69][70]
Lifestyle
Current dietary recommendations to prevent colorectal cancer include increasing the consumption of whole grains, fruits and vegetables, and reducing the intake of red meat and processed meats.[19][71] Higher physical activity is also recommended.[19][72] Physical exercise is associated with a modest reduction in colon but not rectal cancer risk.[73][74] High levels of physical activity reduce the risk of colon cancer by about 21%.[75] Sitting regularly for prolonged periods is associated with higher mortality from colon cancer. The risk is not negated by regular exercise, though it is lowered.[76] The evidence for any protective effect conferred by fiber and fruits and vegetables is, however, poor.[19][77] The risk of colon cancer can be reduced by maintaining a normal body weight.[78]
Medication
Aspirin and celecoxib appear to decrease the risk of colorectal cancer in those at high risk.[79][80] Aspirin is recommended in those who are 50 to 60 years old, do not have an increased risk of bleeding, and are at risk for cardiovascular disease to prevent colorectal cancer.[81] It is not recommended in those at average risk.[82] There is tentative evidence for calcium supplementation, but it is not sufficient to make a recommendation.[83] Vitamin D intake and blood levels are associated with a lower risk of colon cancer.[84][85]
Screening
As more than 80% of colorectal cancers arise from adenomatous polyps, screening for this cancer is effective not only for early detection but also for prevention.[18][86] Diagnosis of cases of colorectal cancer through screening tends to occur 2–3 years before diagnosis of cases with symptoms.[18] Any polyps that are detected can be removed, usually by colonoscopy or sigmoidoscopy, and thus prevent them from turning cancerous. Screening has the potential to reduce colorectal cancer deaths by 60%.[87]
The three main screening tests are colonoscopy, fecal occult blood testing, flexible sigmoidoscopy.[88] Of the three, only sigmoidoscopy cannot screen the right side of the colon where 42% of malignancies are found.[89] Flexible sigmoidoscopy however has the best evidence for decreasing the risk of death from any cause.[90]
Other options may include virtual colonoscopy and stool DNA screening testing.[88] Virtual colonoscopy via a CT scan appears as good as standard colonoscopy for detecting cancers and large adenomas but is expensive, associated with radiation exposure, and cannot remove any detected abnormal growths like standard colonoscopy can.[18]
Fecal occult blood testing (FOBT) of the stool is typically recommended every two years and can be either guaiac based or immunochemical.[18] If abnormal FOBT results are found, participants are typically referred for a follow-up colonoscopy examination. Yearly to every two year FOBT screening reduces colorectal cancer deaths by 16% and among those participating in screening colorectal cancer deaths can be reduced up to 23%, although it has not been proven to reduce all-cause mortality.[91] Immunochemical tests are accurate and do not require dietary or medication changes before testing.[92] The stool DNA screening test looks for biomarkers associated with colorectal cancer and precancerous lesions, including altered DNA and blood hemoglobin. A positive result should be followed by colonoscopy. If used, screening is recommended every 3 years, starting at age 50.[citation needed]
Recommendations
In the United States screening is typically recommended between the age of 50 and 75 years.[5] For those between 76 and 85 years of age the decision to screen should be individualized.[5] A number of screening methods can be used including stool based tests every 3 years, sigmoidoscopy every 5 years and colonoscopy every 10 years. For those at high risk, screenings usually begin at around 40.[18][93] It is unclear which of these two methods is better.[94] Colonoscopy may find more cancers in the first part of the colon but is associated with greater cost and more complications.[94] For people with average risk who have had a high-quality colonoscopy with normal results, the American Gastroenterological Association does not recommend any type of screening in the 10 years following the colonoscopy.[95][96] For people over 75 or those with a life expectancy of less than 10 years, screening is not recommended.[97] It takes about 10 years after screening for one out of a 1000 people to benefit.[98]
In Canada, among those 50 to 75 at normal risk, fecal immunochemical testing or FOBT is recommended every two years or sigmoidoscopy every 10 years.[99] Colonoscopy is less preferred.[99]
Some countries have national colorectal screening programs which offer FOBT screening for all adults within a certain age group, typically starting between age 50 and 60. Examples of countries with organised screening include the United Kingdom,[100] Australia,[101] and the Netherlands.[102]
Treatment
The treatment of colorectal cancer can be aimed at cure or palliation. The decision on which aim to adopt depends on various factors, including the person's health and preferences, as well as the stage of the tumor.[103] When colorectal cancer is caught early, surgery can be curative. However, when it is detected at later stages (for which metastases are present), this is less likely and treatment is often directed at palliation, to relieve symptoms caused by the tumour and keep the person as comfortable as possible.[18]
Surgery


If the cancer is found at a very early stage, it may be removed during a colonoscopy.[6] For people with localized cancer, the preferred treatment is complete surgical removal with adequate margins, with the attempt of achieving a cure. This can either be done by an open laparotomy or sometimes laparoscopically.[18] The colon may then be reconnected or a person may have a colostomy.[6]
If there are only a few metastases in the liver or lungs they may also be removed. Sometimes chemotherapy is used before surgery to shrink the cancer before attempting to remove it. The two most common sites of recurrence of colorectal cancer are the liver and lungs.[18]
Chemotherapy
In both cancer of the colon and rectum, chemotherapy may be used in addition to surgery in certain cases. The decision to add chemotherapy in management of colon and rectal cancer depends on the stage of the disease.
In Stage I colon cancer, no chemotherapy is offered, and surgery is the definitive treatment. The role of chemotherapy in Stage II colon cancer is debatable, and is usually not offered unless risk factors such as T4 tumor or inadequate lymph node sampling is identified. It is also known that the people who carry abnormalities of the mismatch repair genes do not benefit from chemotherapy. For stage III and Stage IV colon cancer, chemotherapy is an integral part of treatment.[18]
If cancer has spread to the lymph nodes or distant organs, which is the case with stage III and stage IV colon cancer respectively, adding chemotherapy agents fluorouracil, capecitabine or oxaliplatin increases life expectancy. If the lymph nodes do not contain cancer, the benefits of chemotherapy are controversial. If the cancer is widely metastatic or unresectable, treatment is then palliative. Typically in this setting, a number of different chemotherapy medications may be used.[18] Chemotherapy drugs for this condition may include capecitabine, fluorouracil, irinotecan, oxaliplatin and UFT.[104] The drugs capecitabine and fluorouracil are interchangeable, with capecitabine being an oral medication while fluorouracil being an intravenous medicine. Some specific regimens used for CRC are FOLFOX, FOLFOXIRI, and FOLFIRI.[105] Antiangiogenic drugs such as bevacizumab are often added in first line therapy. Another class of drugs used in the second line setting are epidermal growth factor receptor inhibitors, of which the two FDA approved ones are cetuximab and panitumumab.[106]
The primary difference in the approach to low stage rectal cancer is the incorporation of radiation therapy. Often, it is used in conjunction with chemotherapy in a neoadjuvant fashion to enable surgical resection, so that ultimately as colostomy is not required. However, it may not be possible in low lying tumors, in which case, a permanent colostomy may be required. Stage IV rectal cancer is treated similar to stage IV colon cancer.
Radiation therapy
While a combination of radiation and chemotherapy may be useful for rectal cancer,[18] its use in colon cancer is not routine due to the sensitivity of the bowels to radiation.[107] Just as for chemotherapy, radiotherapy can be used in the neoadjuvant and adjuvant setting for some stages of rectal cancer.
Immunotherapy
Immunotherapy with immune checkpoint inhibitors has been found to be useful for a type of colorectal cancer with mismatch repair deficiency and microsatellite instability.[108][109] Most people who do improve, however, still worsen after months or years.[109] Other types of colorectal cancer as of 2017 is still being studied.[108][109]
Palliative care
Palliative care is medical care which focuses on treatment of symptoms from serious illness, like cancer, and improving quality of life.[110] Palliative care is recommended for any person who has advanced colon cancer or has significant symptoms.[111]
Involvement of palliative care may be beneficial to improve the quality of life for both the person and his or her family, by improving symptoms, anxiety and preventing admissions to the hospital.[112]
In people with incurable colorectal cancer, palliative care can consist of procedures that relieve symptoms or complications from the cancer but do not attempt to cure the underlying cancer, thereby improving quality of life. Surgical options may include non-curative surgical removal of some of the cancer tissue, bypassing part of the intestines, or stent placement. These procedures can be considered to improve symptoms and reduce complications such as bleeding from the tumor, abdominal pain and intestinal obstruction.[113] Non-operative methods of symptomatic treatment include radiation therapy to decrease tumor size as well as pain medications.[114]
Follow-up
The aims of follow-up are to diagnose, in the earliest possible stage, any metastasis or tumors that develop later, but did not originate from the original cancer (metachronous lesions).
The U.S. National Comprehensive Cancer Network and American Society of Clinical Oncology provide guidelines for the follow-up of colon cancer.[115][116] A medical history and physical examination are recommended every 3 to 6 months for 2 years, then every 6 months for 5 years. Carcinoembryonic antigen blood level measurements follow the same timing, but are only advised for people with T2 or greater lesions who are candidates for intervention. A CT-scan of the chest, abdomen and pelvis can be considered annually for the first 3 years for people who are at high risk of recurrence (for example, those who had poorly differentiated tumors or venous or lymphatic invasion) and are candidates for curative surgery (with the aim to cure). A colonoscopy can be done after 1 year, except if it could not be done during the initial staging because of an obstructing mass, in which case it should be performed after 3 to 6 months. If a villous polyp, a polyp >1 centimeter or high grade dysplasia is found, it can be repeated after 3 years, then every 5 years. For other abnormalities, the colonoscopy can be repeated after 1 year.
Routine PET or ultrasound scanning, chest X-rays, complete blood count or liver function tests are not recommended.[115][116] A 2016 systematic review concluded that more intense surveillance and close follow-up does not provide additional survival benefits in non-metastatic colorectal cancers.[117]
Exercise
Exercise may be recommended in the future as secondary therapy to cancer survivors. In epidemiological studies, exercise may decrease colorectal cancer-specific mortality and all-cause mortality. Results for the specific amounts of exercise needed to observe a benefit were conflicting. These differences may reflect differences in tumour biology and expression of biomarkers. Patients with tumors that lacked CTNNB1 expression (β-catenin), involved in Wnt signalling pathway, required more than 18 Metabolic equivalent (MET) hours per week, a measure of exercise, to observe a reduction in colorectal cancer mortality. The mechanism of how exercise benefits survival may be involved in immune surveillance and inflammation pathways. In clinical studies, a pro-inflammatory response was found in patients with stage II-III colorectal cancer who underwent 2 weeks of moderate exercise after completing their primary therapy. Oxidative balance may be another possible mechanism for benefits observed. A significant decrease in 8-oxo-dG was found in the urine of patients who underwent 2 weeks of moderate exercise after primary therapy. Other possible mechanisms may involve metabolic hormone and sex-steroid hormones, although these pathways may be involved in other types of cancers[118][119]
Another potential biomarker may be p27. Survivors with tumors that expressed p27 and performed greater and equal to 18 MET hours per week were found to have reduced colorectal-cancer mortality survival compared to those with less than 18 MET hours per week. Survivors without p27 expression who exercised were shown to have worse outcomes. The constitutive activation of PI3K/AKT/mTOR pathway may explain the loss of p27 and excess energy balance may up-regulate p27 to stop cancer cells from dividing.[119]
Prognosis
In Europe the five-year survival rate for colorectal cancer is less than 60%. In the developed world about a third of people who get the disease die from it.[18]
Survival is directly related to detection and the type of cancer involved, but overall is poor for symptomatic cancers, as they are typically quite advanced. Survival rates for early stage detection are about five times that of late stage cancers. People with a tumor that has not breached the muscularis mucosa (TNM stage Tis, N0, M0) have a five-year survival rate of 100%, while those with invasive cancer of T1 (within the submucosal layer) or T2 (within the muscular layer) have an average five-year survival rate of approximately 90%. Those with a more invasive tumor yet without node involvement (T3-4, N0, M0) have an average five-year survival rate of approximately 70%. Patients with positive regional lymph nodes (any T, N1-3, M0) have an average five-year survival rate of approximately 40%, while those with distant metastases (any T, any N, M1) have an average five-year survival rate of approximately 5%.[120]
According to American Cancer Society statistics in 2006,[121] over 20% of people with colorectal cancer come to medical attention when the disease is already advanced (stage IV), and up to 25% of this group will have isolated liver metastasis that is potentially resectable. In this selective group, those who undergo curative resection experience a five-year survival outcome in a third of the cases.[122]
Less than 600 genes are linked to outcomes in colorectal cancer.[41] These include both unfavorable genes, where high expression is related to poor outcome, for example the heat shock 70 kDa protein 1 (HSPA1A), and favorable genes where high expression is associated with better survival, for example the putative RNA-binding protein 3 (RBM3).[41]
Epidemiology
Globally more than 1 million people get colorectal cancer every year[18] resulting in about 715,000 deaths as of 2010 up from 490,000 in 1990.[123]
As of 2012[update], it is the second most common cause of cancer in women (9.2% of diagnoses) and the third most common in men (10.0%)[124] with it being the fourth most common cause of cancer death after lung, stomach, and liver cancer.[125] It is more common in developed than developing countries.[126] Globally incidences vary 10-fold with highest rates in Australia, New Zealand, Europe and the US and lowest rates in Africa and South-Central Asia.[127]
United States
Colorectal cancer is the second highest cause of cancer occurrence and death for men and women in the United States combined. An estimated 141,210 cases were diagnosed in 2011.[128]
Based on rates from 2007 to 2009, 4.96% of US men and women born today will be diagnosed with colorectal cancer during their lifetime.[129] From 2005 to 2009, the median age at diagnosis for cancer of the colon and rectum in the US was 69 years of age. Approximately 0.1% were diagnosed under age 20; 1.1% between 20 and 34; 4.0% between 35 and 44; 13.4% between 45 and 54; 20.4% between 55 and 64; 24.0% between 65 and 74; 25.0% between 75 and 84; and 12.0% 85+ years of age. Rates are higher among males (54 per 100,000 c.f. 40 per 100,000 for females).
United Kingdom
In the UK about 41,000 people a year get colon cancer making it the fourth most common type.[130]
Australia
One in 19 men and one in 28 women in Australia will develop colorectal cancer before the age of 75; one in 10 men and one in 15 women will develop it by 85 years of age.[131]
History
Rectal cancer has been diagnosed in an Ancient Egyptian mummy who had lived in the Dakhleh Oasis during the Ptolemaic period.[132]
The Biblical king Jehoram of Judah was recorded in 2 Chronicles 21 to be cursed with an incurable disease of the bowel, leading to his death, due to his supposed evil deeds. Modern scholarship indicates that his condition was most likely colon cancer.[133]
Society and culture
In the United States, March is colorectal cancer awareness month.[87]
Notable cases
- Corazon Aquino, former president of the Philippines[134]
- Pope John Paul II[135]
- Ronald Reagan, actor and former President of the United States[136]
- Harold Wilson, former Prime Minister of the United Kingdom[137]
- Robin Gibb, musician and member of the Bee Gees[138]
- Humayun Ahmed, Bengali writer and film maker[139]
- J. B. S. Haldane, geneticist, polymath, and popular science author[140]
- Stephen Sutton, charity activist[141]
Research
Preliminary in-vitro evidence suggests lactic acid bacteria (e.g., lactobacilli, streptococci or lactococci) may be protective against the development and progression of colorectal cancer through several mechanisms such as antioxidant activity, immunomodulation, promoting programmed cell death, antiproliferative effects, and epigenetic modification of cancer cells.[142]
Large-scale genome sequencing studies have been done to identify mutations in colorectal cancer patients' genome.[143]
The bacteria clostridium novyi-NT, is also being studied.[144]
- Mouse models of colorectal and intestinal cancer
- The Cancer Genome Atlas[43]
- The Colorectal Cancer Atlas integrating genomic and proteomic data pertaining to colorectal cancer tissues and cell lines have been developed.[145]
References
- ^ a b c d e "General Information About Colon Cancer". NCI. May 12, 2014. Archived from the original on July 4, 2014. Retrieved June 29, 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c d e f g h i j k l World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 5.5. ISBN 9283204298.
- ^ a b c d "Colorectal Cancer Prevention (PDQ®)". National Cancer Institute. February 27, 2014. Archived from the original on July 5, 2014. Retrieved June 29, 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b Theodoratou, E; Timofeeva, M; Li, X; Meng, X; Ioannidis, JPA (August 2017). "Nature, Nurture, and Cancer Risks: Genetic and Nutritional Contributions to Cancer". Annual Review of Nutrition (Review). 37: 293-320. doi:10.1146/annurev-nutr-071715-051004. PMID 28826375.
- ^ a b c d e Bibbins-Domingo, Kirsten; Grossman, David C.; Curry, Susan J.; Davidson, Karina W.; Epling, John W.; García, Francisco A. R.; Gillman, Matthew W.; Harper, Diane M.; Kemper, Alex R.; Krist, Alex H.; Kurth, Ann E.; Landefeld, C. Seth; Mangione, Carol M.; Owens, Douglas K.; Phillips, William R.; Phipps, Maureen G.; Pignone, Michael P.; Siu, Albert L. (June 21, 2016). "Screening for Colorectal Cancer". JAMA. 315 (23): 2564–75. doi:10.1001/jama.2016.5989. PMID 27304597.
- ^ a b c d e f g "Colon Cancer Treatment (PDQ®)". NCI. May 12, 2014. Archived from the original on July 5, 2014. Retrieved June 29, 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b "SEER Stat Fact Sheets: Colon and Rectum Cancer". NCI. Archived from the original on June 24, 2014. Retrieved June 18, 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ GBD 2015 Disease and Injury Incidence and Prevalence, Collaborators. (October 8, 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
{{cite journal}}:|first1=has generic name (help)CS1 maint: numeric names: authors list (link) - ^ GBD 2015 Mortality and Causes of Death, Collaborators. (October 8, 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/s0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
{{cite journal}}:|first1=has generic name (help)CS1 maint: numeric names: authors list (link) - ^ "Defining Cancer". National Cancer Institute. Archived from the original on June 25, 2014. Retrieved June 10, 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Thorat, MA; Cuzick, J (December 2013). "Role of aspirin in cancer prevention". Current Oncology Reports. 15 (6): 533–40. doi:10.1007/s11912-013-0351-3. PMID 24114189.
- ^ "Routine aspirin or nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer: recommendation statement". American Family Physician. 76 (1): 109–13. 2007. PMID 17668849. Archived from the original on July 14, 2014.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 1.1. ISBN 9283204298.
- ^ Alpers, David H.; Kalloo, Anthony N.; Kaplowitz, Neil; Owyang, Chung; Powell, Don W. (2008). Yamada, Tadataka (ed.). Principles of clinical gastroenterology. Chichester, West Sussex: Wiley-Blackwell. p. 381. ISBN 978-1-4051-6910-3. Archived from the original on September 28, 2015.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b Astin M, Griffin, T, Neal, RD, Rose, P, Hamilton, W (May 2011). "The diagnostic value of symptoms for colorectal cancer in primary care: a systematic review". The British Journal of General Practice. 61 (586): 231–43. doi:10.3399/bjgp11X572427. PMC 3080228. PMID 21619747.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Adelstein BA, Macaskill, P, Chan, SF, Katelaris, PH, Irwig, L (2011). "Most bowel cancer symptoms do not indicate colorectal cancer and polyps: a systematic review". BMC Gastroenterology. 11: 65. doi:10.1186/1471-230X-11-65. PMC 3120795. PMID 21624112.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b Watson AJ, Collins, PD (2011). "Colon cancer: a civilization disorder". Digestive Diseases. 29 (2): 222–8. doi:10.1159/000323926. PMID 21734388.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j k l m n o p q r s t Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N (2010). "Colorectal cancer". Lancet. 375 (9719): 1030–47. doi:10.1016/S0140-6736(10)60353-4. PMID 20304247.
- ^ a b c d "Colorectal Cancer 2011 Report: Food, Nutrition, Physical Activity, and the Prevention of Colorectal Cancer" (PDF). World Cancer Research Fund & American Institute for Cancer Research. 2011. Archived from the original (PDF) on September 9, 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Lee, I-Min; Shiroma, Eric J; Lobelo, Felipe; Puska, Pekka; Blair, Steven N; Katzmarzyk, Peter T (July 1, 2012). "Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy". The Lancet. 380 (9838): 219–29. doi:10.1016/S0140-6736(12)61031-9. PMC 3645500. PMID 22818936.
- ^ Fedirko V, Tramacere, I, Bagnardi, V, Rota, M, Scotti, L, Islami, F, Negri, E, Straif, K, Romieu, I, La Vecchia, C, Boffetta, P, Jenab, M (September 2011). "Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies". Annals of Oncology. 22 (9): 1958–72. doi:10.1093/annonc/mdq653. PMID 21307158.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Valtin, H (November 2002). ""Drink at least eight glasses of water a day." Really? Is there scientific evidence for "8 x 8"?". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 283 (5): R993-1004. doi:10.1152/ajpregu.00365.2002. PMID 12376390.
- ^ Boleij, Annemarie; van Gelder, Marleen M. H. J.; Swinkels, Dorine W.; Tjalsma, Harold (2011). "Clinical Importance of Streptococcus gallolyticus Infection Among Colorectal Cancer Patients: Systematic Review and Meta-analysis". Clinical Infectious Diseases. 53 (9): 870–878. doi:10.1093/cid/cir609. PMID 21960713.
- ^ Jans, Christoph; Meile, Leo; Lacroix, Christophe; Stevens, Marc J.A. (2015). "Genomics, evolution, and molecular epidemiology of the Streptococcus bovis/Streptococcus equinus complex (SBSEC)". Infection, Genetics and Evolution. 33: 419–436. doi:10.1016/j.meegid.2014.09.017. PMID 25233845.
- ^ a b c Abdulamir, Ahmed S; Hafidh, Rand R; Bakar, Fatimah (2011). "The association of Streptococcus bovis/gallolyticus with colorectal tumors: The nature and the underlying mechanisms of its etiological role". Journal of Experimental & Clinical Cancer Research. 30 (1): 11. doi:10.1186/1756-9966-30-11. ISSN 1756-9966. PMC 3032743. PMID 21247505.
{{cite journal}}: CS1 maint: unflagged free DOI (link) This article incorporates text by Ahmed S Abdulamir, Rand R Hafidh, and Fatimah Abu Bakar available under the CC BY 2.0 license.
This article incorporates text by Ahmed S Abdulamir, Rand R Hafidh, and Fatimah Abu Bakar available under the CC BY 2.0 license.
- ^ Jawad N, Direkze, N, Leedham, SJ (2011). "Inflammatory bowel disease and colon cancer". Recent Results in Cancer Research. Recent Results in Cancer Research. 185: 99–115. doi:10.1007/978-3-642-03503-6_6. ISBN 978-3-642-03502-9. PMID 21822822.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hu T, Li LF, Shen J, Zhang L, Cho CH (2015). "Chronic inflammation and colorectal cancer: the role of vascular endothelial growth factor". Current pharmaceutical design. 21 (21): 2960–2967. doi:10.2174/1381612821666150514104244. PMID 26004415.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Xie J, Itzkowitz, SH (2008). "Cancer in inflammatory bowel disease". World Journal of Gastroenterology. 14 (3): 378–89. doi:10.3748/wjg.14.378. PMC 2679126. PMID 18200660.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b c d Triantafillidis JK, Nasioulas, G, Kosmidis, PA (July 2009). "Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies". Anticancer Research. 29 (7): 2727–37. PMID 19596953.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Juhn E, Khachemoune, A (2010). "Gardner syndrome: skin manifestations, differential diagnosis and management". American Journal of Clinical Dermatology. 11 (2): 117–22. doi:10.2165/11311180-000000000-00000. PMID 20141232.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Half E, Bercovich, D, Rozen, P (2009). "Familial adenomatous polyposis". Orphanet Journal of Rare Diseases. 4: 22. doi:10.1186/1750-1172-4-22. PMC 2772987. PMID 19822006.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Möslein G, Pistorius S, Saeger H, Schackert HK (February 2003). "Preventive surgery for colon cancer in familial adenomatous polyposis and hereditary nonpolyposis colorectal cancer syndrome". Langenbecks Arch. Surg. 388 (1): 9–16. doi:10.1007/s00423-003-0364-8. PMID 12690475.
- ^ Stein, Ulrike; Walther, Wolfgang; Arlt, Franziska; Schwabe, Holger; Smith, Janice; Fichtner, Iduna; Birchmeier, Walter; Schlag, Peter M (2008). "MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis". Nature Medicine. 15 (1): 59–67. doi:10.1038/nm.1889. PMID 19098908.
- ^ Stein U (2013) MACC1 – a novel target for solid cancers. Expert Opin Ther Targets
- ^ Schuebel, Kornel E.; Chen, Wei; Cope, Leslie; Glöckner, Sabine C.; Suzuki, Hiromu; Yi, Joo-Mi; Chan, Timothy A.; Van Neste, Leander; Van Criekinge, Wim; van den Bosch, Sandra; van Engeland, Manon; Ting, Angela H.; Jair, Kamwing; Yu, Wayne; Toyota, Minoru; Imai, Kohzoh; Ahuja, Nita; Herman, James G.; Baylin, Stephen B. (2007). "Comparing the DNA Hypermethylome with Gene Mutations in Human Colorectal Cancer". PLoS Genetics. 3 (9): e157. doi:10.1371/journal.pgen.0030157. PMC 1988850. PMID 17892325. Archived from the original on September 8, 2015. Retrieved August 28, 2015.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M (1993). "Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis". Nature. 363 (6429): 558–61. doi:10.1038/363558a0. PMID 8505985.
- ^ Srikumar Chakravarthi; Baba Krishnan; Malathy Madhavan (1999). "Apoptosis and expression of p53 in colorectal neoplasms". Indian J. Med. Res. 86 (7): 95–102.
- ^ Khalek FJ, Gallicano GI, Mishra L (May 2010). "Colon Cancer Stem Cells". Gastrointest. Cancer Res. (Suppl 1): S16–S23. PMC 3047031. PMID 21472043.
- ^ a b c d Markowitz SD, Bertagnolli MM (December 2009). "Molecular Origins of Cancer: Molecular Basis of Colorectal Cancer". N. Engl. J. Med. 361 (25): 2449–60. doi:10.1056/NEJMra0804588. PMC 2843693. PMID 20018966.
- ^ Mehlen P, Fearon ER (August 2004). "Role of the dependence receptor DCC in colorectal cancer pathogenesis". J. Clin. Oncol. 22 (16): 3420–8. doi:10.1200/JCO.2004.02.019. PMID 15310786.
- ^ a b c Uhlen, Mathias; Zhang, Cheng; Lee, Sunjae; Sjöstedt, Evelina; Fagerberg, Linn; Bidkhori, Gholamreza; Benfeitas, Rui; Arif, Muhammad; Liu, Zhengtao (August 18, 2017). "A pathology atlas of the human cancer transcriptome". Science. 357 (6352): eaan2507. doi:10.1126/science.aan2507. ISSN 0036-8075. PMID 28818916.
- ^ Vogelstein, B; Kinzler, KW (2004). "Cancer genes and the pathways they control". Nature Medicine. 10 (8): 789–99. doi:10.1038/nm1087. PMID 15286780.
- ^ a b c Muzny, DM.; Bainbridge, MN.; Chang, K.; Dinh, HH.; Drummond, JA.; Fowler, G.; Kovar, CL.; Lewis, LR.; Morgan, MB.; Newsham, Irene F.; Reid, Jeffrey G.; Santibanez, Jireh; Shinbrot, Eve; Trevino, Lisa R.; Wu, Yuan-Qing; Wang, Min; Gunaratne, Preethi; Donehower, Lawrence A.; Creighton, Chad J.; Wheeler, David A.; Gibbs, Richard A.; Lawrence, Michael S.; Voet, Douglas; Jing, Rui; Cibulskis, Kristian; Sivachenko, Andrey; Stojanov, Petar; McKenna, Aaron; Lander, Eric S.; Gabriel, Stacey (July 2012). "Comprehensive molecular characterization of human colon and rectal cancer". Nature. 487 (7407): 330–7. doi:10.1038/nature11252. PMC 3401966. PMID 22810696.
- ^ Slaughter, DP; Southwick, HW; Smejkal, W (1953). "Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin". Cancer. 6 (5): 963–8. doi:10.1002/1097-0142(195309)6:5<963::AID-CNCR2820060515>3.0.CO;2-Q. PMID 13094644.
- ^ Giovannucci, E; Ogino, S (September 21, 2005). "DNA methylation, field effects, and colorectal cancer". Journal of the National Cancer Institute. 97 (18): 1317–9. doi:10.1093/jnci/dji305. PMID 16174847.
- ^ a b Bernstein, C; Prasad, AR; Nfonsam, V; Bernstein, H. (2013). "DNA Damage, DNA Repair and Cancer". In Chen, Clark (ed.). New Research Directions in DNA Repair. InTech. ISBN 978-953-51-1114-6.
- ^ Bernstein, C; Bernstein, H; Payne, CM; Dvorak, K; Garewal, H (2008). "Field defects in progression to gastrointestinal tract cancers". Cancer Letters. 260 (1–2): 1–10. doi:10.1016/j.canlet.2007.11.027. PMC 2744582. PMID 18164807.
- ^ Nguyen, H; Loustaunau, C; Facista, A; Ramsey, L; Hassounah, N; Taylor, H; Krouse, R; Payne, CM; Tsikitis, VL; Goldschmid, S; Banerjee, B; Perini, RF; Bernstein, C (2010). "Deficient Pms2, ERCC1, Ku86, CcOI in field defects during progression to colon cancer". Journal of Visualized Experiments (41): 1931. doi:10.3791/1931. PMC 3149991. PMID 20689513. 28 minute video Archived September 10, 2017, at the Wayback Machine
- ^ Rubin, H (2011). "Fields and field cancerization: The preneoplastic origins of cancer: Asymptomatic hyperplastic fields are precursors of neoplasia, and their progression to tumors can be tracked by saturation density in culture". BioEssays. 33 (3): 224–31. doi:10.1002/bies.201000067. PMID 21254148.
- ^ Tsao, JL; Yatabe, Y; Salovaara, R; Järvinen, HJ; Mecklin, JP; Aaltonen, LA; Tavaré, S; Shibata, D (2000). "Genetic reconstruction of individual colorectal tumor histories". Proceedings of the National Academy of Sciences of the United States of America. 97 (3): 1236–41. doi:10.1073/pnas.97.3.1236. PMC 15581. PMID 10655514.
- ^ a b Vogelstein, B; Papadopoulos, N; Velculescu, VE; Zhou, S; Diaz Jr, LA; Kinzler, KW (2013). "Cancer genome landscapes". Science. 339 (6127): 1546–58. doi:10.1126/science.1235122. PMC 3749880. PMID 23539594.
- ^ "Etiologic field effect: reappraisal of the field effect concept in cancer predisposition and progression". Mod. Pathol. 28: 14–29. 2014. doi:10.1038/modpathol.2014.81. PMC 4265316. PMID 24925058.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ Balaguer F, Link A, Lozano JJ, et al. (August 2010). "Epigenetic silencing of miR-137 is an early event in colorectal carcinogenesis". Cancer Res. 70 (16): 6609–18. doi:10.1158/0008-5472.CAN-10-0622. PMC 2922409. PMID 20682795.
- ^ Deng, G; Kakar, S; Kim, YS (2011). "MicroRNA-124a and microRNA-34b/c are frequently methylated in all histological types of colorectal cancer and polyps, and in the adjacent normal mucosa". Oncology Letters. 2 (1): 175–180. doi:10.3892/ol.2010.222. PMC 3412539. PMID 22870149.
- ^ Grady, WM; Parkin, RK; Mitchell, PS; Lee, JH; Kim, YH; Tsuchiya, KD; Washington, MK; Paraskeva, C; Willson, JK; Kaz, AM; Kroh, EM; Allen, A; Fritz, BR; Markowitz, SD; Tewari, M (2008). "Epigenetic silencing of the intronic microRNA hsa-miR-342 and its host gene EVL in colorectal cancer". Oncogene. 27 (27): 3880–8. doi:10.1038/onc.2008.10. PMID 18264139.
- ^ Kanwal, R; Gupta, S (2012). "Epigenetic modifications in cancer". Clinical Genetics. 81 (4): 303–11. doi:10.1111/j.1399-0004.2011.01809.x. PMC 3590802. PMID 22082348.
- ^ Baldassarre, G; Battista, S; Belletti, B; Thakur, S; Pentimalli, F; Trapasso, F; Fedele, M; Pierantoni, G; Croce, CM; Fusco, A (2003). "Negative regulation of BRCA1 gene expression by HMGA1 proteins accounts for the reduced BRCA1 protein levels in sporadic breast carcinoma". Molecular and Cellular Biology. 23 (7): 2225–38. doi:10.1128/MCB.23.7.2225-2238.2003. PMC 150734. PMID 12640109.
- ^ a b Schnekenburger, M; Diederich, M (2012). "Epigenetics Offer New Horizons for Colorectal Cancer Prevention". Current Colorectal Cancer Reports. 8 (1): 66–81. doi:10.1007/s11888-011-0116-z. PMC 3277709. PMID 22389639.
- ^ Jacinto FV, Esteller M (July 2007). "Mutator pathways unleashed by epigenetic silencing in human cancer". Mutagenesis. 22 (4): 247–53. doi:10.1093/mutage/gem009. PMID 17412712. Archived from the original on September 4, 2015.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Lahtz C, Pfeifer GP (February 2011). "Epigenetic changes of DNA repair genes in cancer". J. Mol. Cell. Biol. 3 (1): 51–8. doi:10.1093/jmcb/mjq053. PMC 3030973. PMID 21278452. Archived from the original on October 16, 2015.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Bernstein C, Nfonsam V, Prasad AR, Bernstein H (March 2013). "Epigenetic field defects in progression to cancer". World J. Gastrointest. Oncol. 5 (3): 43–9. doi:10.4251/wjgo.v5.i3.43. PMC 3648662. PMID 23671730. Archived from the original on December 15, 2013.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Weerakkody, Yuranga; Gaillard, Frank. "Colorectal carcinoma". Radiopaedia.org. Retrieved September 13, 2014.
- ^ Di Como, Joseph A. (October 2015). "Adenosquamous carcinoma of the colon and rectum: a population based clinical outcomes study involving 578 patients from the Surveillance Epidemiology and End Result (SEER) database (1973-2010)". Journal of the American College of Surgeons. 221 (4): 56. doi:10.1016/j.jamcollsurg.2015.08.044.
- ^ "Moderately differentiated adenocarcinoma (colon)". pathologyatlas.ro. Archived from the original on January 26, 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Sostres C, Gargallo CJ, Lanas A (February 2014). "Aspirin, cyclooxygenase inhibition and colorectal cancer". World J. Gastrointest. Pharmacol. Ther. 5 (1): 40–9. doi:10.4292/wjgpt.v5.i1.40. PMC 3944468. PMID 24605250.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Metastatic Cancer". National Cancer Institute. February 6, 2017. Archived from the original on April 4, 2017. Retrieved April 5, 2017.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Mitrovic, B.; Schaeffer, D. F.; Riddell, R. H.; Kirsch, R. (2012). "Tumor budding in colorectal carcinoma: Time to take notice". Modern Pathology. 25 (10): 1315–25. doi:10.1038/modpathol.2012.94. PMID 22790014.
- ^ Parkin, D. M.; Boyd, L.; Walker, L. C. (December 6, 2011). "16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010". British Journal of Cancer. 105 (S2): S77–S81. doi:10.1038/bjc.2011.489. ISSN 0007-0920. PMC 3252065. PMID 22158327. Archived from the original on April 9, 2016.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Searke, David (2006). Cancer Epidemiology and Prevention (3 ed.). Oxford University Press. p. 809. ISBN 9780199747979. Archived from the original on September 28, 2015.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Rennert, Gad (2007). Cancer Prevention. Springer. p. 179. ISBN 9783540376965. Archived from the original on October 3, 2015.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Campos FG, Logullo Waitzberg, AG, Kiss, DR, Waitzberg, DL, Habr-Gama, A, Gama-Rodrigues, J (January 2005). "Diet and colorectal cancer: current evidence for etiology and prevention". Nutricion Hospitalaria. 20 (1): 18–25. PMID 15762416.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pérez-Cueto, Federico J. A.; Verbeke, Wim (April 1, 2012). "Consumer implications of the WCRF's permanent update on colorectal cancer". Meat Science. 90 (4): 977–978. doi:10.1016/j.meatsci.2011.11.032. ISSN 1873-4138. PMID 22196090.
- ^ Harriss DJ, Atkinson, G, Batterham, A, George, K, Cable, NT, Reilly, T, Haboubi, N, Renehan, AG, Colorectal Cancer, Lifestyle, Exercise And Research, Group (September 2009). "Lifestyle factors and colorectal cancer risk (2): a systematic review and meta-analysis of associations with leisure-time physical activity". Colorectal Disease. 11 (7): 689–701. doi:10.1111/j.1463-1318.2009.01767.x. PMID 19207713.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Robsahm TE, Aagnes B, Hjartåker A, Langseth H, Bray FI, Larsen IK (November 2013). "Body mass index, physical activity, and colorectal cancer by anatomical subsites: A systematic review and meta-analysis of cohort studies". Eur. J. Cancer Prev. 22 (6): 492–505. doi:10.1097/CEJ.0b013e328360f434. PMID 23591454.
- ^ Kyu, Hmwe H; Bachman, Victoria F; Alexander, Lily T; Mumford, John Everett; Afshin, Ashkan; Estep, Kara; Veerman, J Lennert; Delwiche, Kristen; Iannarone, Marissa L; Moyer, Madeline L; Cercy, Kelly; Vos, Theo; Murray, Christopher J L; Forouzanfar, Mohammad H (August 9, 2016). "Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013". BMJ. 354: i3857. doi:10.1136/bmj.i3857. PMC 4979358. PMID 27510511.
- ^ Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, Alter DA (2015). "Sedentary Time and Its Association With Risk for Disease Incidence, Mortality, and Hospitalization in Adults: A Systematic Review and Meta-analysis". Annals of Internal Medicine. 162 (2): 123–32. doi:10.7326/M14-1651. PMID 25599350.
- ^ Doyle VC, Logullo Waitzberg, AG, Kiss, DR, Waitzberg, DL, Habr-Gama, A, Gama-Rodrigues, J (May 2007). "Nutrition and colorectal cancer risk: a literature review". Gastroenterology Nursing. 30 (3): 178–82, quiz 182–3. doi:10.1097/01.SGA.0000278165.05435.c0. PMID 17568255.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lauby-Secretan, B; Scoccianti, C; Loomis, D; Grosse, Y; Bianchini, F; Straif, K; International Agency for Research on Cancer Handbook Working, Group (August 25, 2016). "Body Fatness and Cancer – Viewpoint of the IARC Working Group". The New England Journal of Medicine. 375 (8): 794–798. doi:10.1056/nejmsr1606602. PMID 27557308.
- ^ Cooper K, Squires, H, Carroll, C, Papaioannou, D, Booth, A, Logan, RF, Maguire, C, Hind, D, Tappenden, P (June 2010). "Chemoprevention of colorectal cancer: systematic review and economic evaluation". Health Technology Assessment. 14 (32): 1–206. doi:10.3310/hta14320. PMID 20594533.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Emilsson, L; Holme, Ø; Bretthauer, M; Cook, NR; Buring, JE; Løberg, M; Adami, HO; Sesso, HD; Gaziano, MJ; Kalager, M (January 2017). "Systematic review with meta-analysis: the comparative effectiveness of aspirin vs. screening for colorectal cancer prevention". Alimentary pharmacology & therapeutics. 45 (2): 193–204. doi:10.1111/apt.13857. PMID 27859394.
- ^ Bibbins-Domingo, Kirsten (April 12, 2016). "Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement". Annals of Internal Medicine. 164: 836–45. doi:10.7326/M16-0577. PMID 27064677.
- ^ Agency for Healthcare Research and Quality. "Aspirin or Nonsteroidal Anti-inflammatory Drugs for the Primary Prevention of Colorectal Cancer". United States Department of Health & Human Services. Archived from the original on January 5, 2016.
2010/2011
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Weingarten MA, Zalmanovici, A, Yaphe, J (2008). Weingarten, Michael Asher MA (ed.). "Dietary calcium supplementation for preventing colorectal cancer and adenomatous polyps". Cochrane Database of Systematic Reviews (1): CD003548. doi:10.1002/14651858.CD003548.pub4. PMID 18254022.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ma Y, Zhang, P, Wang, F, Yang, J, Liu, Z, Qin, H (2011). "Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies". Journal of Clinical Oncology. 29 (28): 3775–82. doi:10.1200/JCO.2011.35.7566. PMID 21876081.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Yin L, Grandi, N, Raum, E, Haug, U, Arndt, V, Brenner, H (July 2011). "Meta-analysis: Serum vitamin D and colorectal adenoma risk". Preventive Medicine. 53 (1–2): 10–6. doi:10.1016/j.ypmed.2011.05.013. PMID 21672549.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "What Can I Do to Reduce My Risk of Colorectal Cancer?". Centers for Disease Control and Prevention. April 2, 2014. Archived from the original on February 26, 2015. Retrieved March 5, 2015.
{{cite journal}}: Cite journal requires|journal=(help); Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b He J, Efron, JE (2011). "Screening for colorectal cancer". Advances in Surgery. 45: 31–44. doi:10.1016/j.yasu.2011.03.006. PMID 21954677.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b "Colorectal cancer screening". www.dynamed.com. Archived from the original on March 25, 2017. Retrieved March 24, 2017.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Siegel RL, Ward EM, Jemal A (March 2012). "Trends in Colorectal Cancer Incidence Rates in the United States by Tumor Location and Stage, 1992–2008". Cancer Epidemiology, Biomarkers & Prevention. 21 (3): 411–6. doi:10.1158/1055-9965.EPI-11-1020. PMID 22219318. Archived from the original on January 29, 2013. Retrieved September 16, 2012.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Swartz, AW; Eberth, JM; Josey, MJ; Strayer, SM (August 22, 2017). "Re-analysis of All-Cause Mortality in the U.S. Preventive Services Task Force 2016 Evidence Report on Colorectal Cancer Screening". Annals of Internal Medicine. doi:10.7326/M17-0859. PMID 28828493.
- ^ Hewitson, P; Glasziou, P; Watson, E; Towler, B; Irwig, L (June 2008). "Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update". The American Journal of Gastroenterology. 103 (6): 1541–9. doi:10.1111/j.1572-0241.2008.01875.x. PMID 18479499.
- ^ Lee, Jeffrey K.; Liles, Elizabeth G.; Bent, Stephen; Levin, Theodore R.; Corley, Douglas A. (February 4, 2014). "Accuracy of Fecal Immunochemical Tests for Colorectal Cancer". Annals of Internal Medicine. 160 (3): 171–181. doi:10.7326/M13-1484.
- ^ "Screening for Colorectal Cancer". U.S. Preventive Services Task Force. 2008. Archived from the original on February 7, 2015.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b Brenner, H.; Stock, C.; Hoffmeister, M. (April 9, 2014). "Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies". BMJ. 348 (apr09 1): g2467–g2467. doi:10.1136/bmj.g2467.
- ^ American Gastroenterological Association. "Five Things Physicians and Patients Should Question" (PDF). Choosing Wisely: an initiative of the ABIM Foundation. American Gastroenterological Association. Archived from the original (PDF) on August 9, 2012. Retrieved August 17, 2012.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Winawer, S; Fletcher, R; Rex, D; Bond, J; Burt, R; Ferrucci, J; Ganiats, T; Levin, T; Woolf, S; Johnson, D; Kirk, L; Litin, S; Simmang, C; Gastrointestinal Consortium, Panel (February 2003). "Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence". Gastroenterology. 124 (2): 544–60. doi:10.1053/gast.2003.50044. PMID 12557158.
- ^ Qaseem A, Denberg TD, Hopkins RH Jr, et al. (2012). "Screening for Colorectal Cancer: A Guidance Statement From the American College of Physicians". Annals of Internal Medicine. 156 (5): 378–386. doi:10.7326/0003-4819-156-5-201203060-00010. PMID 22393133. Archived from the original on October 1, 2012.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Tang, V; Boscardin, WJ; Stijacic-Cenzer, I; Lee, SJ (April 16, 2015). "Time to benefit for colorectal cancer screening: survival meta-analysis of flexible sigmoidoscopy trials". BMJ. 350: h1662. doi:10.1136/bmj.h1662. PMC 4399600. PMID 25881903.
- ^ a b Bacchus, CM; Dunfield, L; Connor Gorber, S; Holmes, NM; Birtwhistle, R; Dickinson, JA; Lewin, G; Singh, H; Klarenbach, S; Mai, V; Tonelli, M; Canadian Task Force on Preventive Health, Care (February 22, 2016). "Recommendations on screening for colorectal cancer in primary care". CMAJ : Canadian Medical Association Journal. 188: 340–8. doi:10.1503/cmaj.151125. PMID 26903355.
- ^ "NHS Bowel Cancer Screening Programme". cancerscreening.nhs.uk. Archived from the original on November 29, 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Home – Bowel Cancer Australia". bowelcanceraustralia.org. Archived from the original on December 24, 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Bevolkingsonderzoek darmkanker". rivm.nl. Archived from the original on December 17, 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Stein A, Atanackovic, D, Bokemeyer, C (September 2011). "Current standards and new trends in the primary treatment of colorectal cancer". European Journal of Cancer. 47 (Suppl 3): S312–4. doi:10.1016/S0959-8049(11)70183-6. PMID 21943995.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Chemotherapy of metastatic colorectal cancer". Prescrire International. 19 (109): 219–24. October 2010. PMID 21180382.
- ^ "Current status of pharmacological treatment of colorectal cancer". World J Gastrointest Oncol. 6 (6): 177–83. 2014. doi:10.4251/wjgo.v6.i6.177. PMC 4058725. PMID 24936228.
{{cite journal}}: Cite uses deprecated parameter|authors=(help)CS1 maint: unflagged free DOI (link) - ^ Shaib, W; Mahajan, R; El-Rayes, B (2013). "Markers of resistance to anti-EGFR therapy in colorectal cancer". Journal of Gastrointestinal Oncology. 4 (3): 308–18. doi:10.3978/j.issn.2078-6891.2013.029. PMC 3712296. PMID 23997942.
- ^ authors, editors, Vincent T. DeVita Jr., Theodore S. Lawrence, Steven A. Rosenberg ; associate scientific advisors, Robert A. Weinberg, Ronald A. DePinho ; with 421 contributing (2008). DeVita, Hellman, and Rosenberg's cancer : principles & practice of oncology (8th ed.). Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins. p. 1258. ISBN 978-0-7817-7207-5. Archived from the original on September 19, 2015.
{{cite book}}:|first=has generic name (help); Unknown parameter|deadurl=ignored (|url-status=suggested) (help)CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ a b Boland, PM; Ma, WW (May 11, 2017). "Immunotherapy for Colorectal Cancer". Cancers. 9 (5). doi:10.3390/cancers9050050. PMID 28492495.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c Syn, Nicholas L; Teng, Michele W L; Mok, Tony S K; Soo, Ross A. "De-novo and acquired resistance to immune checkpoint targeting". The Lancet Oncology. 18 (12): e731–e741. doi:10.1016/s1470-2045(17)30607-1.
- ^ "Palliative or Supportive Care". American Cancer Society. Archived from the original on August 21, 2014. Retrieved August 20, 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "ASCO Provisional Clinical Opinion: The Integration of Palliative Care into Standard Oncology Care". ASCO. Archived from the original on August 21, 2014. Retrieved 20 August 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Higginson, IJ; Evans, CJ (September–October 2010). "What is the evidence that palliative care teams improve outcomes for cancer patients and their families?". Cancer Journal. 16 (5): 423–35. doi:10.1097/PPO.0b013e3181f684e5. PMID 20890138.
- ^ Wasserberg N, Kaufman HS (December 2007). "Palliation of colorectal cancer". Surg. Oncol. 16 (4): 299–310. doi:10.1016/j.suronc.2007.08.008. PMID 17913495.
- ^ Amersi F, Stamos MJ, Ko CY (July 2004). "Palliative care for colorectal cancer". Surg. Oncol. Clin. N. Am. 13 (3): 467–77. doi:10.1016/j.soc.2004.03.002. PMID 15236729.
- ^ a b "National Comprehensive Cancer Network" (PDF). nccn.org. Archived from the original (PDF) on March 25, 2009.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b Desch CE, Benson AB 3rd, Somerfield MR, et al.; American Society of Clinical Oncology (2005). "Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline". J. Clin. Oncol. 23 (33): 8512–9. doi:10.1200/JCO.2005.04.0063. PMID 16260687.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ Jeffery, M; Hickey, BE; Hider, PN; See, AM (November 24, 2016). "Follow-up strategies for patients treated for non-metastatic colorectal cancer". The Cochrane database of systematic reviews. 11: CD002200. doi:10.1002/14651858.CD002200.pub3. PMID 27884041.
- ^ Betof AS, Dewhirst MW, Jones LW (March 2013). "Effects and potential mechanisms of exercise training on cancer progression: A translational perspective". Brain Behav. Immun. 30: S75-87. doi:10.1016/j.bbi.2012.05.001. PMC 3638811. PMID 22610066.
- ^ a b Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM (May 2012). "Physical activity, biomarkers, and disease outcomes in cancer survivors: A systematic review". J. Natl. Cancer Inst. 104 (11): 815–40. doi:10.1093/jnci/djs207. PMC 3465697. PMID 22570317.
- ^ Box 3-1, Page 107 in: Elizabeth D Agabegi; Agabegi, Steven S. (2008). Step-Up to Medicine (Step-Up Series). Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 0-7817-7153-6.
- ^ [1] Archived September 25, 2006, at the Wayback Machine
- ^ Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M (April 2006). "Surgical resection of hepatic metastases from colorectal cancer: A systematic review of published studies". Br. J. Cancer. 94 (7): 982–99. doi:10.1038/sj.bjc.6603033. PMC 2361241. PMID 16538219.
- ^ Lozano R, Naghavi M, Foreman K, et al. (December 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2095–128. doi:10.1016/S0140-6736(12)61728-0. PMID 23245604.
- ^ World Cancer Report 2014. International Agency for Research on Cancer, World Health Organization. 2014. ISBN 978-92-832-0432-9.
- ^ WHO (February 2010). "Cancer". World Health Organization. Archived from the original on December 29, 2010. Retrieved January 5, 2011.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Merika E, Saif, MW, Katz, A, Syrigos, K, Morse, M (September 2010). "Review. Colon cancer vaccines: an update". In Vivo. 24 (5): 607–28. PMID 20952724.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Colorectal Cancer Incidence, Mortality and Prevalence Worldwide in 2008 — Summary Archived October 17, 2012, at the Wayback Machine. Available from: Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. (2010) GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet] Archived May 8, 2011, at the Wayback Machine. Lyon, France: International Agency for Research on Cancer. Accessed on 11 Oct 2012.
- ^ "American Journal of Health Education". American Journal of Health Education. 43 (4): 194. 2012.
- ^ Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations). Bethesda, MD: National Cancer Institute. Archived from the original on September 3, 2012.
{{cite book}}:|author=has generic name (help); Unknown parameter|deadurl=ignored (|url-status=suggested) (help)CS1 maint: multiple names: authors list (link) Based on November 2011 SEER data submission, posted to the SEER web site, 2012. - ^ "Bowel cancer | About bowel cancer | Cancer Research UK". www.cancerresearchuk.org. Archived from the original on March 9, 2017. Retrieved May 12, 2017.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Cancer in Australia: an Overview, 2014. Cancer series No 90. Cat. No. CAN 88. Canberra: Australian Institute of Health and Welfare. 2014. ISBN 978-1-74249-677-1.
- ^ Rehemtulla, Alnawaz (December 2010). "Dinosaurs and Ancient Civilizations: Reflections on the Treatment of Cancer". Neoplasia. 12 (12): 957–968. doi:10.1593/neo.101588. PMC 3003131. PMID 21170260.
- ^ Louba, Liubov (December 2004). "Colorectal carcinoma that afflicted King Jehoram". Minerva Med. 95 (6): 557–561. PMID 15785440.
- ^ [2][dead link]
- ^ "Pope John Paul II". ABC News Online. Archived from the original on December 18, 2009.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Reagan turns 90". BBC News: Americas. February 6, 2001. Archived from the original on March 3, 2016.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Goodman, Geoffrey (July 1, 2005). "Harold Wilson | Politics". The Guardian. London. Archived from the original on December 4, 2013. Retrieved April 10, 2014.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Klein, Sarah (April 23, 2012). "12 Famous Faces Touched By Colorectal Cancer". Huffington Post. Archived from the original on April 25, 2012.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Humayun Ahmed dies". Bdnews24.com. July 19, 2012. Archived from the original on July 21, 2012. Retrieved July 19, 2012.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Krishna Dronamraju (May 2010). "J.B.S. Haldane's Last Years: His Life and Work in India [1957–1964]". Genetics. 185 (1): 5–10. doi:10.1534/genetics.110.116632. PMC 2870975. PMID 20516291.
- ^ "Cancer fundraiser Stephen Sutton dies aged 19". BBC News. Archived from the original on May 14, 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Zhong L, Zhang X, Covasa M (June 2014). "Emerging roles of lactic acid bacteria in protection against colorectal cancer". World J. Gastroenterol. 20 (24): 7878–86. doi:10.3748/wjg.v20.i24.7878. PMC 4069315. PMID 24976724.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
- ^ Liu, Y; Zhang, X; Han, C; Wan, G; Huang, X; Ivan, C; Jiang, D; Rodriguez-Aguayo, C; Lopez-Berestein, G; Rao, PH; Maru, DM; Pahl, A; He, X; Sood, AK; Ellis, LM; Anderl, J; Lu, X (April 30, 2015). "TP53 loss creates therapeutic vulnerability in colorectal cancer". Nature. 520 (7549): 697–701. doi:10.1038/nature14418. PMC 4417759. PMID 25901683.
- ^ Mone, Amy. "New Treatment and Research". The Johns Hopkins Kimmel Cancer Center. Archived from the original on May 14, 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Colorectal Cancer Atlas". Archived from the original on January 13, 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)