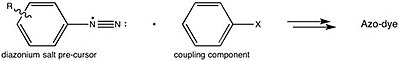
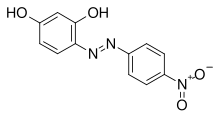
Azo violet
| |
| Names | |
|---|---|
| IUPAC name
4-[(E)-(4-Nitrophenyl)diazenyl]benzene-1,3-diol
| |
| Other names
(E)-4-[(4-Nitrophenyl)diazenyl]benzene-1,3-diol
4-(4-Nitrophenyl)azobenzene-1,3-diol Magneson I p-Nitrophenylazoresorcinol 4-Nitrophenylazoresorcinol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.735 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H9N3O4 | |
| Molar mass | 259.318 g mol−1 |
| Appearance | dark red to brown crystalline powder |
| Density | 1.45 g/cm3 |
| 1 g/L H2O; 4 g/L Ethanol | |
| Hazards | |
| Flash point | 261.7 °C (503.1 °F; 534.8 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
| Azo violet (pH indicator) | ||
| below pH 11.0 | above pH 13.0 | |
| 11.0 | ⇌ | 13.0 |
Azo violet (Magneson I;[1] p-nitrobenzeneazoresorcinol) is an azo compound with the chemical formula C12H9N3O4. It is used commercially as a violet dye and experimentally as a pH indicator, appearing yellow below pH 11, and violet above pH 13.[2] It also turns deep blue in the presence of magnesium salt in a slightly alkaline, or basic, environment.[3][4] Azo violet may also be used to test for the presence of ammonium ions[citation needed]. The color of ammonium chloride or ammonium hydroxide solution will vary depending upon the concentration of azo violet used. Magneson I is used to test Be also; it produces an orange-red lake with Be(II) in alkaline medium.[5]
Properties[edit]

The intense color from which the compound gets its name results from irradiation and subsequent excitation and relaxation of the extended π electron system across the R-N=N-R' linked phenols. Absorption of these electrons falls in the visible region of the electromagnetic spectrum. Azo violet's intense indigo color (λmax 432 nm) approximates Pantone R: 102 G: 15 B: 240.
Synthesis[edit]
Azo violet can be synthesised by reacting 4-nitroaniline with nitrous acid (generated in situ with an acid and a nitrite salt) to produce a diazonium intermediate. This is then reacted with resorcinol, dissolved in a sodium hydroxide solution, via an azo coupling reaction.
This is consistent with the generalized strategy for preparing azo dyes.
Reactivity[edit]
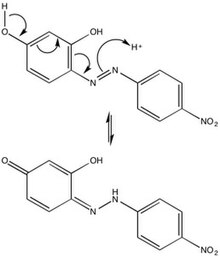
The chemical character of azo violet may be attributed to its azo group (-N=N-), six-membered rings, and hydroxyl side groups. Due to steric repulsions, azo violet is most stable in the trans-configuration, but isomerization of azo dyes by irradiation is not uncommon. The para-position tautomerization of azo violet provides mechanical insight into the behavior of the compound in an acidic environment, and thus its use as a basic pH indicator.
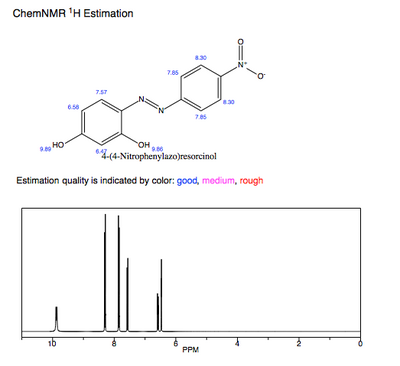
The predicted 1H-NMR of pure azo violet shows the hydroxyl protons as the most deshielded and acidic protons. The participation of these hydroxyl groups' electron-donation to the conjugated π system likewise influences azo violet's λmax and pKa value.
References[edit]
- ^ "Magneson | C12H9N3O4 | ChemSpider". www.chemspider.com. Retrieved 2022-07-30.
- ^ "Azo Violet 25GM from Cole-Parmer". Cole-Parmer. Archived from the original on 28 October 2016. Retrieved 28 October 2016.
- ^ Feigl, F.; Anger, V. (2012-12-02). Spot Tests in Inorganic Analysis. Elsevier. ISBN 9780444597984.
- ^ Gopalan, R. (2009-01-01). Inorganic Chemistry for Undergraduates. Universities Press. ISBN 9788173716607.
- ^ Gopalan, R. (2009). Inorganic Chemistry for Undergraduates. Universities Press. ISBN 978-81-7371-660-7.