One-pot synthesis
In chemistry a one-pot synthesis is a strategy to improve the efficiency of a chemical reaction whereby a reactant is subjected to successive chemical reactions in just one reactor. This is much desired by chemists because avoiding a lengthy separation process and purification of the intermediate chemical compounds would save time and resources while increasing chemical yield.
An example of a one-pot synthesis is the total synthesis of tropinone or the Gassman indole synthesis.
A sequential one-pot synthesis with reagents added to a reactor one at a time and without work-up is also called a telescoping synthesis.
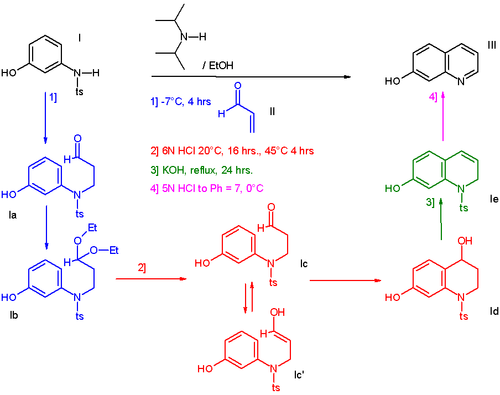
In one such procedure [1] the reaction of 3-N-tosylaminophenol I with acrolein II affords a hydroxyl substituted quinoline III through 4 sequential steps without workup of the intermediate products [2]:
References
- ^ One-Pot Preparation of 7-Hydroxyquinoline Mark Cameron, R. Scott Hoerrner, James M. McNamara, Margaret Figus, and Scott Thomas Org. Process Res. Dev.; 2006; 10(1) pp 149 - 152; (Communication to Editor) DOI: Abstract
- ^ The addition of acrolein (blue) is a Michael reaction catalyzed by N,N-diisopropylamine, the presence of ethanol converts the aldehyde group to an acetal but this process is reversed when hydrochloric acid is introduced (red). The enolate reacts as an electrophile in a Friedel-Crafts reaction with ring-closure. The alcohol group is eliminated in presence of potassium hydroxide (green) and when in the final step the reaction medium is neutralized to pH 7 (magenta) the tosyl group is eliminated as well.
