Polyphenol


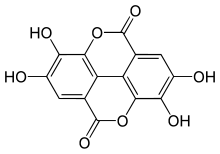
Polyphenols[1][2] (noun, pronunciation of the singular /pɒliˈfiːnəl/[3] or /pɒliˈfɛnəl/, also known as polyhydroxyphenols) are a structural class of mainly natural, but also synthetic or semisynthetic, organic chemicals characterized by the presence of large multiples of phenol structural units. The number and characteristics of these phenol structures underlie the unique physical, chemical, and biological (metabolic, toxic, therapeutic, etc.) properties of particular members of the class. Examples include tannic acid (image at right) and ellagitannin (image below). The historically important chemical class of tannins is a subset of the polyphenols.[1][4]
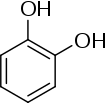
The name derives from the ancient Greek word πολύς (polus, meaning "many, much") and the word phenol which refers to a chemical structure formed by attaching to an aromatic benzenoid (phenyl) ring, an hydroxyl (-OH) group akin to that found in alcohols (hence the "-ol" suffix). The term polyphenol appears to have been in use since 1894.[3]
Definition of the term polyphenol

Original "WBSSH" definition of polyphenols
The earliest widely accepted definition of polyphenols, the White–Bate-Smith–Swain–Haslam (WBSSH) definition,[5] was offered and justified by natural product and organic chemist Edwin Haslam and co-workers, based on the earlier natural products research of Edgar Charles Bate-Smith, Anthony Swain, and Theodore White that characterized specific structural characteristics common to plant phenolics used in tanning (i.e., the tannins).[6] The WBSSH describes the polyphenol class as:
- generally moderately water-soluble compounds
- with molecular weight of 500–4000 Da
- with >12 phenolic hydroxyl groups
- with 5–7 aromatic rings per 1000 Da
where the limits to these ranges are somewhat flexible.[1][5] The definition further states that polyphenols display unique physical and chemical behaviors related to their high molecular weights and profusion of phenolic substructures—precipitation of proteins and particular amine-containing organics (e.g., particular alkaloid natural products), and formation of particular metal complexes (e.g., intense blue-black iron(III) complexes).
Proposed Quideau definition of polyphenols
The need to clarify the definition of 'polyphenols' in the light of the extensive research into this large substance class and of increasingly ambiguous use of the polyphenol term led Stéphane Quideau, Bordeaux 1 University, France, to offer a definition not given formal status by IUPAC:[2]
- The term "polyphenol" should be used to define compounds exclusively derived from the shikimate/phenylpropanoid and/or the polyketide pathway, featuring more than one phenolic unit and deprived of nitrogen-based functions.
Structurally, this definition continues to steer the definition away from exclusively man-made structures without corresponding natural products, and explicitly excludes monophenolic structures (man-made or naturally occurring) and their derivatives, e.g., phenyl esters, methyl phenyl ethers and O-phenyl glycosides. This definition departs from the WBSSH definition in terms of physicochemical behavior, with its lack of reference to solubility, precipitation, and complexation phenomena.
The gallic acid dimer, ellagic acid (M.W. 302, right), a molecule at the core of naturally occurring phenolic compounds of varying sizes, is itself not a polyphenol by the WBSSH definition, but is by the Quideau definition. The raspberry ellagitannin (M.W. ~2450),[7] on the other hand, with its 14 gallic acid moieties (most in ellagic acid-type components), and more than 40 phenolic hydroxyl groups, meets the criteria of both definitions of a polyphenol. Other examples of compounds that fall under both the WBSSH and Quideau definitions include the black tea antioxidant theaflavin-3-gallate shown below, and the hydrolyzable tannin, tannic acid, shown above.

Defining chemical reactions of the polyphenol class
Individual polyphenols engage in reactions related to both their core phenolic structures, their linkages, and types of glycosides they form. Standard phenolic reactions include ionization (which contributes to solubility and complexation), oxidations to ortho- and para-quinones (which contributes to antioxidant characteristics), and underlying aromatic transformations related to the presence of the phenolic hydroxyl (see phenol image above); reactions related to their linkages include nucleophilic additions, and oxidative and hydrolytic bond cleavages.[8] In addition, as noted above, a traditional feature of polyphenols was their ability to form particular, characteristic metal complexes.[5]
Chemical structure and synthesis
Structural features
As opposed to smaller phenols, polyphenols are often larger molecules (macromolecules) deposited in cell vacuoles. The upper molecular weight limit for small molecules is about 800 Daltons, which allows for the possibility to rapidly diffuse across cell membranes so that they can reach intracellular sites of action or remain as pigments once the cell senesces. Hence, many larger polyphenols are biosynthesized in-situ from smaller polyphenols to nonhydrolyzable tannins and remain undiscovered in the plant matrix. Most polyphenols contain repeating phenolic moieties of pyrocatechol, resorcinol, pyrogallol, and phloroglucinol connected by esters (hydrolyzable tannins) or more stable C-C bonds (nonhydrolyzable condensed tannins). Proanthocyanidins are mostly polymeric units of catechin and epicatechin. Catechol and resorcinol (benzenediol) types of polyphenols have two, and pyrogallol and phloroglucinol (benzenetriol) types have three phenolic hydroxyl groups, respectively, though mixing of these types within polyphenols is also possible. The phenolic substructures arise from various biosynthetic pathways (WBSSH definition), especially phenylpropanoid and polyketide branches aimed at plant and related secondary metabolites (both definitions).
 |
 |
 |
 |

Polyphenols always have heteroatom substituents other than hydroxyl groups; ether and ester linkages are common, as are various carboxylic acid derivatives (see theaflavin gallate image); ester linkages are common in the hydrolyzable tannins. Apart from simple heteroatom links, the carbon frameworks can become complex, e.g., various carbon-carbon bond linkages join hydrolytically labile esters and ethers as common in non-hydrolyzable condensed tannins.


In these, diverse biosynthetic steps abound: the seven-atom ring (seven-membered ring) appearing in theaflavin structure above is an example of a "carbocycle" that is of a nonbenzenoid aromatic tropolone type. In addition, there are periodic occurrences of:
- benzopyrans and normal and C-glucoside derivatives (figure at right)—e.g. in condensed, complex and hydrolyzable tannins such as in stenophyllanin A, acutissimin B, mongolicain A, stenophynin A, mongolicanin, and mongolicin B,
- various biaryls and triaryls (e.g., biphenyls), see further figure at right,
- spiro-type structures as illustrated at right, e.g., in mongolicain A,
- furanoid, pyrone, and other heterocycles,
- (diaryl)methyl structures,
- pyrans and dioxins, etc.[4]
Because of the preponderance of saccharide-derived core structures (e.g., see tannic acid image above), as well as spiro- and other structure types, natural chiral (stereo) centers abound.
Chemical synthesis
True polyphenols from the tannin and other WBSSH types are routinely biosynthesized in the natural sources from which they derive; their 'chemical' syntheses (using standard "bench" organic chemical methods) were somewhat limited until the first decade of the new millennium because these syntheses involve challenging regioselectivity and stereoselectivity issues.[9] Early work focused on the achiral synthesis of phenolic-related components of polyphenols in the late 70's,[10] and the Nelson and Meyers synthesis of the permethyled derivative of the ubiquitous diphenic acid core of ellagitannins in 1994[11] followed by stereoselective synthesis of more complex permethylated structures such as a (+)-tellimagrandin II derivative by Lipshutz and coworkers in the same year,[12] and Itoh and coworker's synthesis of a permethylated pedunculagin with particular attention to axial symmetry issues in 1996.[13] The total synthesis of a fully unmasked polyphenol, that of the ellagitannin tellimagrandin I, was a diastereoselective sequence reported in 1994 by Feldman, Ensel and Minard.[14]
Further total syntheses of deprotected polyphenols that followed were led by the Feldman group, for instance in Feldman and Lawlor's synthesis of the ellagitannin, coriariin A and other tannin relatives.[15] Khanbabaee and Grosser accomplished a relatively efficient total synthesis of pedunculagin in 2003.[16][17]
Work proceeded with focus on enantioselective total syntheses, e.g., on atroposelective syntheses of axially chiral biaryl polyphenols,[18][19] with recent further important work including controlled assembly of a variety of polyphenols according to integrated strategies, such as in syntheses of extended series of procyanidins (oligomeric catechins) by various groups[20] and of resveratrol polyphenols by the Snyder group at Columbia that included the diverse carasiphenols B and C, ampelopsins G and H, and nepalensinol B.[21][22] A biomimetic synthesis, and the first formal total synthesis 5-O-Desgalloyl-epi-punicacortein A, a further ellagitannin in its C-glucosyl (C-glucoside subclass), has also recently been accomplished.[23] The novel strategies and methods referred to in these recent examples helped to open the field of polyphenol chemical synthesis to an unprecedented degree.[22]
Chemical properties and uses
Chemical properties
Polyphenols are molecules owing their UV/Vis absorptivity to aromatic structures with large conjugated systems of pi electron configurations; they also have autofluorescence properties, especially lignin and the phenolic part of suberin.[24]
They are reactive species toward oxidation.[25] ABTS may be used to characterise polyphenol oxidation products.[26]
Polyphenols also characteristically possess a significant binding affinity for proteins, which can lead to the formation of soluble and insoluble protein-polyphenol complexes.[27]
Chemical uses
Some polyphenols are traditionally used as dyes. For instance, in the Indian subcontinent, the pomegranate peel, high in tannins and other polyphenols, or its juice, is employed in the dyeing of non-synthetic fabrics.[28]
Polyphenols, especially tannins, were used traditionally for tanning leather and today also as precursors in green chemistry[29] notably to produce plastics or resins by polymerisation with[30] or without the use of formaldehyde[31] or adhesives for particleboards.[32] The aims are generally to make use of plant residues from grape, olive (called pomaces) or pecan shells left after processing.[33]
Cashew nut shell liquid (CNSL) is an important phenolic raw material containing mostly cardol, cardanol and anacardic acid. Strictly speaking not a polyphenol, it is used mainly in polymer-based industries for friction linings, paints, varnishes, laminating resins, rubber compounding resins, polyurethane based polymers, surfactants, epoxy resins and wood preservatives.[34]
Pyrogallol and pyrocatechin are among the oldest photographic developers.[35]: 25
Biology
Biological role in plants
Both natural phenols and the larger polyphenols play important roles in the ecology of most plants. Their effects in plant tissues can be divided into the following categories:[36]
- Release and suppression of growth hormones such as auxin.
- UV screens to protect against ionizing radiation and to provide coloration (plant pigments).
- Deterrence of herbivores (sensory properties).
- Prevention of microbial infections (phytoalexins).[37]
- Signaling molecules in ripening and other growth processes.
Occurrence in nature
The most abundant polyphenols are the condensed tannins, found in virtually all families of plants. Larger polyphenols are often concentrated in leaf tissue, the epidermis, bark layers, flowers and fruits but also play important roles in the decomposition of forest litter, and nutrient cycles in forest ecology. Absolute concentrations of total phenols in plant tissues differ widely depending on the literature source, type of polyphenols and assay; they are in the range of 1-25% total natural phenols and polyphenols, calculated with reference to the dry green leaf mass.[38]
High levels of polyphenols in some woods can explain their natural preservation against rot.[39]
Flax and Myriophyllum spicatum (a submerged aquatic plant) secrete polyphenols that are involved in allelopathic interactions.[40][41]
Polyphenols are also found in animals. In arthropods such as insects[42] and crustaceans[43] polyphenols play a role in epicuticle hardening (sclerotization). The hardening of the cuticle is due to the presence of a polyphenol oxidase.[44] In crustaceans, there is a second oxidase activity leading to cuticle pigmentation.[45] There is apparently no polyphenol tanning occurring in arachnids cuticle.[46]
Metabolism
Biosynthesis and metabolism
Polyphenols incorporate smaller parts and building blocks from simpler natural phenols, which originate from the phenyl propanoid pathway for the phenolic acids or the shikimic acid pathway for gallotannins and analogs. Flavonoids and caffeic acid derivatives are biosynthesized from phenyl alanine and malonyl-CoA. Complex gallotannins develop through the in-vitro oxidation of 1,2,3,4,6-pentagalloyl-glucose or dimerization processes resulting in hydrolyzable tannins. For anthocyanidins, precursors of the condensed tannin biosynthesis, dihydroflavonol reductase and leucoanthocyanidin reductase (LAR) are crucial enzymes with subsequent addition of catechin and epicatechin moieties for larger, non-hydrolyzable tannins.[47]
The glycosylated form develops from glucosyltransferase activity and increases the solubility of polyphenols.[48]
Polyphenol oxidase (PPO) is an enzyme that catalyses the oxidation of o-diphenols to produce o-quinones. It is the rapid polymerisation of o-quinones to produce black, brown or red polyphenolic pigments that is the cause of fruit browning. In insects, PPO serves for the cuticle hardening.[49]
Laccase is a major enzyme that initiates the cleavage of hydrocarbon rings, which catalyzes the addition of a hydroxyl group to phenolic compounds. This enzyme can be found in fungi like Panellus stipticus, organisms able to break down lignin, a complex aromatic polymer in wood that is highly resistant to degradation by conventional enzyme systems.
Anthracyclines, hypericin and phenolic lipids[50] are derived from polyketides cyclisation.[51]
Content in food
Generally foods contain complex mixtures of polyphenols.[52] According to a 2005 review on polyphenols:
The most important food sources are commodities widely consumed in large quantities such as fruit and vegetables, green tea, black tea, red wine, coffee, chocolate, olives, and extra virgin olive oil. Herbs and spices, nuts and algae are also potentially significant for supplying certain polyphenols. Some polyphenols are specific to particular food (flavanones in citrus fruit, isoflavones in soya, phloridzin in apples); whereas others, such as quercetin, are found in all plant products such as fruit, vegetables, cereals, leguminous plants, tea, and wine.[52]
Some polyphenols are considered antinutrients, compounds that interfere with the absorption of essential nutrients, especially iron and other metal ions, but also by binding to digestive enzymes and other proteins, particularly in ruminants.[53]
Phenolic and carotenoid compounds with antioxidant properties in vegetables have been found to be retained significantly better through steaming than through frying.[54]
Polyphenols in wine, beer and various nonalcoholic juice beverages can be removed using finings, substances that are usually added at or near the completion of the processing of brewing.
Potential health effects
Many polyphenolic extracts, for example from grape skin, grape seeds, olive pulp and maritime pine bark are sold as ingredients in functional foods, dietary supplements and cosmetics without any legal health claims. Some of them have self-affirmed GRAS status in the US. There are no recommended Dietary Reference Intake levels established for polyphenols.[55]
The diverse structures of phenolic compounds prohibit broad statements about their specific health effects. Further, many purported health claims for specific polyphenol-enriched foods remain unproven.[56] Many of the phytoestrogens are dietary polyphenols with measurable affinities to estrogen receptors, and positive or negative health effects on humans and livestock.[57]
Compared with the effects of polyphenols in vitro, the effects in vivo, although the subject of ongoing research, are limited and vague. The reasons for this are 1) the absence of validated in vivo biomarkers, especially for inflammation or carcinogenesis; 2) long-term studies failing to demonstrate effects with a mechanism of action, specificity or efficacy; and 3) invalid applications of high, unphysiological test concentrations in the in vitro studies, which are subsequently irrelevant for the design of in vivo experiments.[58]
A review of studies on the bioavailability of polyphenols published in 2010 found that "definitive conclusions on bioavailability of most polyphenols are difficult to obtain and further studies are necessary."[52]
Traditional medicine
Many herbal teas contain soluble polyphenols, and their efficacy is often attributed to astringent substances.[59] In the Ayurveda system of medicine for example, the pomegranate has extensively been used as a source of traditional remedies.[28]
Research techniques
Sensory properties
With respect to food and beverages, the cause of astringency is not fully understood, but it is measured chemically as the ability of a substance to precipitate proteins.[60]
A review published in 2005 found that astringency increases and bitterness decreases with the mean degree of polymerization. For water-soluble polyphenols, molecular weights between 500 and 3000 were reported to be required for protein precipitation. However, smaller molecules might still have astringent qualities likely due to the formation of unprecipitated complexes with proteins or cross-linking of proteins with simple phenols that have 1,2-dihydroxy or 1,2,3-trihydroxy groups.[61] Flavonoid configurations can also cause significant differences in sensory properties, e.g. epicatechin is more bitter and astringent than its chiral isomer catechin. In contrast, hydroxycinnamic acids do not have astringent qualities, but are bitter.[62]
Analysis
The analysis techniques are those of phytochemistry: extraction, isolation, structural elucidation,[63] then quantification.
Extraction
Extraction of polyphenols[64] can be performed using a solvent like water, hot water, methanol, methanol/formic acid, methanol/water/acetic or formic acid etc. Liquid liquid extraction can be also performed or countercurrent chromatography. Solid phase extraction can also be made on C18 sorbent cartridges. Other techniques are ultrasonic extraction, heat reflux extraction, microwave-assisted extraction,[65] critical carbon dioxide,[66] pressurized liquid extraction[67] or use of ethanol in an immersion extractor.[68] The extraction conditions (temperature, extraction time, ratio of solvent to raw material, solvent and concentrations) have to be optimized.
Mainly found in the fruit skins and seeds, high levels of polyphenols may reflect only the measured extractable polyphenol (EPP) content of a fruit which may also contain non-extractable polyphenols. Black tea contains high amounts of polyphenol and makes up for 20% of its weight.[69]
Concentration can be made by ultrafiltration.[70] Purification can be achieved by preparative chromatography.
Analysis techniques

Phosphomolybdic acid is used as a reagent for staining phenolics in thin layer chromatography. Polyphenols can be studied by spectroscopy, especially in the ultraviolet domain, by fractionation or paper chromatography. They can also be analysed by chemical characterisation.
Instrumental chemistry analyses include separation by high performance liquid chromatography (HPLC), and especially by reversed-phase liquid chromatography (RPLC), can be coupled to mass spectrometry. Purified compounds can be identified by the mean of nuclear magnetic resonance.
Microscopy analysis
The DMACA reagent is an histological dye specific to polyphenols used in microscopy analyses. The autofluorescence of polyphenols can also be used, especially for localisation of lignin and suberin.
Quantification
Polyphenolic content can be quantified separation/isolation by volumetric titration. An oxidizing agent, permanganate, is used to oxidize known concentrations of a standard tannin solution, producing a standard curve. The tannin content of the unknown is then expressed as equivalents of the appropriate hydrolyzable or condensed tannin.[71]
Some methods for quantification of total polyphenol content are based on colorimetric measurements.[72] Some tests are relatively specific to polyphenols (for instance the Porter's assay). Total phenols (or antioxidant effect) can be measured using the Folin-Ciocalteu reaction. Results are typically expressed as gallic acid equivalents. Polyphenols are seldom evaluated by antibody technologies.[73]
Other tests measure the antioxidant capacity of a fraction. Some make use of the ABTS radical cation which is reactive towards most antioxidants including phenolics, thiols and vitamin C.[74] During this reaction, the blue ABTS radical cation is converted back to its colorless neutral form. The reaction may be monitored spectrophotometrically. This assay is often referred to as the Trolox equivalent antioxidant capacity (TEAC) assay. The reactivity of the various antioxidants tested are compared to that of Trolox, which is a vitamin E analog.
Other antioxidant capacity assays which use Trolox as a standard include the diphenylpicrylhydrazyl (DPPH), oxygen radical absorbance capacity (ORAC),[75] ferric reducing ability of plasma (FRAP)[76] assays or inhibition of copper-catalyzed in vitro human low-density lipoprotein oxidation.[77]
New methods including the use of biosensors can help monitor the content of polyphenols in food.[78]
Quantitation results produced by the mean of diode array detector-coupled HPLC are generally given as relative rather than absolute values as there is a lack of commercially available standards for all polyphenolic molecules.
See also
- Polyphenolic proteins
- List of antioxidants in food
- List of phytochemicals in food
- Nutrition
- Phytochemistry
- Secondary metabolites
- Oligostilbenoids
References
- ^ a b c Quideau, S. P.; Deffieux, D.; Douat-Casassus, C. L.; Pouységu, L. (2011). "Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis". Angewandte Chemie International Edition. 50 (3): 586–621. doi:10.1002/anie.201000044.
- ^ a b Quideau S (2011). "Why bother with polyphenols?". Groupe Polyphenols. Retrieved 26 March 2014.
- ^ a b Polyphenol on www.merriam-webster.com online dictionary
- ^ a b Nonaka, G. (1989). "Isolation and structure elucidation of tannins" (PDF). Pure & Appl. Chem. 61 (3): 357–360. doi:10.1351/pac198961030357.
- ^ a b c Haslam, E.; Cai, Y. (1994). "Plant polyphenols (vegetable tannins): Gallic acid metabolism". Natural Product Reports. 11 (1): 41–66. doi:10.1039/NP9941100041. PMID 15206456.
- ^ Practical Polyphenolics, Edwin Haslam, 1998, ISBN 0-521-46513-3
- ^ Cardiovascular disease and phytochemicals. Anonymous. C. Hamilton et al.
- ^ Drynan, J.W.; Clifford, M.N.; Obuchowicz, J.; Kuhnert, N. (2010). "The chemistry of low molecular weight black tea polyphenols". Nat. Prod. Rep. 27: 417–462. doi:10.1039/b912523j.
- ^ Krohn, K.; Ahmed, I.; John, M.; Letzel, M. C.; Kuck, D. (2010). "Stereoselective Synthesis of Benzylated Prodelphinidins and Their Diastereomers with Use of the Mitsunobu Reaction in the Preparation of Their Gallocatechin Precursors". Eur. J. Org. Chem. 2010: 2544–2554. doi:10.1002/ejoc.201000053.
- ^ Botha JJ et al. (1978). Direct synthesis, structure and absolute configuration of biflavonoids from black wattle bark (mimosa) extract. J. Chem. Soc. Chem. Commun. ;700 (1978)
- ^ Nelson, TD; Meyers, AI (1994). "A Rapid Total Synthesis of an Ellagitannin [sic]". J. Org. Chem. 59 (9): 2577–2580. doi:10.1021/jo00088a046.
- ^ Lipshutz, BH; Liu, ZP; Kayser, F (1994). "Cyanocuprate-Mediated Intramolecular Biaryl Couplings Applied to an Ellagitannin - Synthesis of (+)-O-Permethyltellimagrandin II". Tet. Lett. 35 (31): 5567–5570. doi:10.1016/s0040-4039(00)77248-0.
- ^ Itoh, T; Chika, J; Shirakami, S; et al. (1996). "Synthesis of trideca-O-methyl-alpha-pedunculagin. Diastereo-favoritism studies on intramolecular ester-cyclization of axially chiral diphenic acids with carbohydrate core". J. Org. Chem. 61 (11): 3700–3705. doi:10.1021/jo950969j.
- ^ Feldman, KS; Ensel, SM (1994). "Ellagitannin chemistry. Preparative and mechanistic studies of the biomimetic oxidative coupling of galloyl esters". J. Amer. Chem. Soc. 116 (8): 3357–3366. doi:10.1021/ja00087a022.
- ^ Feldman, KS; Lawlor, MD; Sahasrabudhe, K (2000). "Ellagitannin chemistry. Evolution of a three-component coupling strategy for the synthesis of the dimeric ellagitannin coriariin A and a dimeric gallotannin analogue". J. Org. Chem. 65 (23): 8011–8019. doi:10.1021/jo0010936.
- ^ Khanbabaee, K; Grosser, M (2003). "An efficient total synthesis of pedunculagin by using a twofold intramolecular double esterification strategy". Eur. J. Org. Chem. 11: 2128–2131.
- ^ Feldman, KS (2004). "Recent progress in ellagitannin chemistry". Phytochemistry. 66 (17): 1984–2000. doi:10.1016/j.phytochem.2004.11.015.
- ^ Bringmann, G; Gulder, T; Gulder, TAM; et al. (2011). "Total Synthesis of Axially Chiral Biaryl Natural Products". Chem. Revs. 111 (2): 563–639. doi:10.1021/cr100155e.
- ^ Pouysegu, L.; Deffieux, D.; Gaelle, G. Malik; et al. (2011). "Synthesis of ellagitannin natural products". Nat. Prod. Rep. 28 (5): 853–874. doi:10.1039/c0np00058b.
- ^ Kozikowski, AP; Tückmantel, W; Boettcher, G; LJ Romanczyk, Jr (2003). "J. Org. Chem. 68: 1641–1658; K Ohmori, T Shono, Y Hatakoshi, T Yano, T. and K Suzuki (2011), Integrated Synthetic Strategy for Higher Catechin Oligomers". Angew. Chem. Int. Ed. 50: 4862–4867.
- ^ Snyder, S.A.; Gollner, A.; Chiriac, M.I. (2011). "Regioselective reactions for programmable resveratrol oligomer synthesis". Nature. 474: 461–466. doi:10.1038/nature10197. PMC 3179663. PMID 21697944.
- ^ a b Quideau, S. (2011). "Organic chemistry: Triumph for unnatural synthesis". Nature. 474: 459–460. doi:10.1038/474459a. PMID 21697943.
- ^ Deffieux, D.; Natangelo, A.; Malik, G.; Pouységu, L.; Charris, J.; Quideau, S. (2011). "First and biomimetic total synthesis of a member of the C-glucosidic subclass of ellagitannins, 5-O-desgalloyl-epi-punicacortein A.". Chem. Comm. 47: 1628–1630. doi:10.1039/c0cc04007j.
- ^ Force A, See to Act.FLUORESCENCE AND POLYPHENOLS retrieved July 31, 2013.
- ^ Santos, M.A; Bonilla Venceslada, J.L; Martin Martin, A; Garcia Garcia, I (2005). "Estimating the selectivity of ozone in the removal of polyphenols from vinasse". Journal of chemical technology and biotechnology. 80 (4): 433–438. doi:10.1002/jctb.1222. INIST 16622840.
- ^ Osman, A. M.; Wong, K. K. Y.; Fernyhough, A. (2006). "ABTS radical-driven oxidation of polyphenols: Isolation and structural elucidation of covalent adducts". Biochemical and Biophysical Research Communications. 346 (1): 321–329. doi:10.1016/j.bbrc.2006.05.118. PMID 16756947.
- ^ Papadopoulou, A. and Frazier, R.A. (2004) "Characterization of protein-polyphenol interactions." Trends in Food Science & Technology, 15 (3-4). pp. 186-190. [ISSN 0924-2244 http://centaur.reading.ac.uk/13092/]
- ^ a b K. K. Jindal; R. C. Sharma (2004). Recent trends in horticulture in the Himalayas. Indus Publishing. ISBN 81-7387-162-0.
... bark of tree and rind of fruit is commonly used in ayurveda ... also used for dyeing ...
- ^ Polshettiwar, Vivek; Varma, Rajender S. (2008). "Greener and expeditious synthesis of bioactive heterocycles using microwave irradiation". Pure and Applied Chemistry. 80 (4): 777–90. doi:10.1351/pac200880040777.
- ^ Hillis, W. E.; Urbach, G. (1959). "Reaction of polyphenols with formaldehyde". Journal of Applied Chemistry. 9 (12): 665–673. doi:10.1002/jctb.5010091207.
- ^ Fukuoka, Tokuma; Uyama, Hiroshi; Kobayashi, Shiro (2003). "Synthesis of Ultrahigh Molecular Weight Polyphenols by Oxidative Coupling". Macromolecules. 36 (22): 8213–5. doi:10.1021/ma034803t.
- ^ Pizzi, A.; Valenezuela, J.; Westermeyer, C. (1994). "Low formaldehyde emission, fast pressing, pine and pecan tannin adhesives for exterior particleboard". Holz als Roh- und Werkstoff. 52 (5): 311–5. doi:10.1007/BF02621421.
- ^ Aizpurua-Olaizola, Oier; Ormazabal, Markel; Vallejo, Asier; Olivares, Maitane; Navarro, Patricia; Etxebarria, Nestor; Usobiaga, Aresatz (1 January 2015). "Optimization of Supercritical Fluid Consecutive Extractions of Fatty Acids and Polyphenols from Vitis Vinifera Grape Wastes". Journal of Food Science. 80 (1): E101–E107. doi:10.1111/1750-3841.12715. ISSN 1750-3841.
- ^ Mathew Obichukwu EDOGA, Labake FADIPE, Rita Ngozi EDOGA (2006). "Extraction of Polyphenols from Cashew Nut Shell". Leonardo Electronic Journal of Practices and Technologies, p. 107-112. ISSN 1583-1078
- ^ Stephen G. Anchell; Bill Troop. The Film Developing Cookbook. ISBN 978-0240802770.
{{cite book}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ V. Lattanzio et al. (2006). Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects (and references therein). Phytochemistry: Advances in Research, 23-67. ISBN 81-308-0034-9.
- ^ Huber, B; Eberl, L; Feucht, W; Polster, J (2003). "Influence of polyphenols on bacterial biofilm formation and quorum-sensing". Z. Naturforsch. C. 58 (11–12): 879–84. doi:10.1515/znc-2003-11-1224. PMID 14713169.
- ^ Stephan Hättenschwiler and Peter M. Vitousek. TREE vol. 15, no. 6 June 2000. PII: S0169-5347(00)01861-9
- ^ Hart, John H.; Hillis, W. E. (1974). "Inhibition of wood-rotting fungi by stilbenes and other polyphenols in Eucalyptus sideroxylon". Phytopathology. 64 (7): 939–48. doi:10.1094/Phyto-64-939.
- ^ Popa, V; Dumitru, M; Volf, I; Anghel, N (2008). "Lignin and polyphenols as allelochemicals". Industrial Crops and Products. 27 (2): 144–9. doi:10.1016/j.indcrop.2007.07.019.
- ^ Nakai, S (2000). "Myriophyllum spicatum-released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa". Water Research. 34 (11): 3026–32. doi:10.1016/S0043-1354(00)00039-7.
- ^ Wigglesworth, V. B. (1988). "The source of lipids and polyphenols for the insect cuticle: The role of fat body, oenocytes and oenocytoids". Tissue and Cell. 20 (6): 919–932. doi:10.1016/0040-8166(88)90033-X. PMID 18620248.
- ^ Dennell, R. (1947). "The Occurrence and Significance of Phenolic Hardening in the Newly Formed Cuticle of Crustacea decapoda". Proceedings of the Royal Society B: Biological Sciences. 134 (877): 485–503. doi:10.1098/rspb.1947.0027.
- ^ Locke, M.; Krishnan, N. (1971). "The distribution of phenoloxidases and polyphenols during cuticle formation". Tissue and Cell. 3 (1): 103–126. doi:10.1016/S0040-8166(71)80034-4. PMID 18631545.
- ^ Krishnan, G. "Phenolic Tanning and Pigmentation of the Cuticle in Carcinus maenas". Quarterly Journal of Microscopical Science. 92: 333–342.
- ^ Krishnan, G. "The Epicuticle of an Arachnid, Palamneus swammerdami". Quarterly Journal of Microscopical Science. 95: 371–381.
- ^ G. Tanner et al. (Aug 2003). Proanthocyanidin Biosynthesis in Plants. The Journal of Biological Chemistry, 278, 31647-31656. 10.1074/jbc.M302783200
- ^ Krasnow, M. N.; Murphy, T. M. (2004). "Polyphenol Glucosylating Activity in Cell Suspensions of Grape (Vitis vinifera)". Journal of Agricultural and Food Chemistry. 52 (11): 3467–3472. doi:10.1021/jf035234r. PMID 15161217.
- ^ Malek, S. R. A. (1961). "Polyphenols and their quinone derivatives in the cuticle of the desert locust, Schistocerca gregaria (Forskål)". Comparative Biochemistry and Physiology. 2: 35–77. doi:10.1016/0010-406X(61)90071-8.
- ^ A Review of the Hypothetical Biogenesis and Regulation of Hypericin synthesis via the Polyketide Pathway in Hypericum perforatum and Experimental Methods Proposed to Evaluate the Hypothesis. Loren W. Walker, Portland State University , May, 1999
- ^ Hertweck, C. (2009). "The Biosynthetic Logic of Polyketide Diversity". Angewandte Chemie International Edition. 48 (26): 4688–4716. doi:10.1002/anie.200806121.
- ^ a b c D'Archivio, M; et al. (2010). "Bioavailability of the Polyphenols: Status and Controversies". Int. J. Mol. Sci. 11 (4): 1321–1342. doi:10.3390/ijms11041321. PMC 2871118. PMID 20480022.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ L. Mennen; et al. (January 2005). "Risks and Safety of Polyphenol Consumption". Am J Clin Nutr. 81 (1): 3265–3295. PMID 15640498.
- ^ Miglio C, Chiavaro E, Visconti A, Fogliano V, Pellegrini N (2008). "Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables". J Agric Food Chem. 56 (1): 139–47. doi:10.1021/jf072304b. PMID 18069785.
- ^ E. Watson (Nov 2012). FOOD navigator-usa.com. Who has self-affirmed GRAS?
- ^ Halliwell B (2007). "Dietary polyphenols: Good, bad, or indifferent for your health?". Cardiovasc Res. 73 (2): 341–347. doi:10.1016/j.cardiores.2006.10.004. PMID 17141749.
- ^ Izabela Wocławek-Potocka, Chiara Mannelli, Dorota Boruszewska, Ilona Kowalczyk-Zieba, Tomasz Waśniewski, and Dariusz J. Skarżyński, "Diverse Effects of Phytoestrogens on the Reproductive Performance: Cow as a Model," International Journal of Endocrinology, vol. 2013, Article ID 650984, 15 pages, 2013. article doi:10.1155/2013/650984
- ^ Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies" Am J Clin Nutr 81:(1) 243S-5S, 2005. Dietary polyphenols and health: Proceedings of the 1st International Conference on Polyphenols and Health
- ^ "Traditional herbal medicines--the role of polyphenols". Planta Med. 55 (1): 1–8. February 1989. doi:10.1055/s-2006-961764. PMID 2654977.
- ^ Staff, Sensory Society. Basic Tastes: Astringency
- ^ Lesschaeve I, Noble AC (2005). "Polyphenols: factors influencing their sensory properties and their effects on food and beverage preferences". Am J Clin Nutr. 81 (1 Suppl): 330S-335S. PMID 15640499.
- ^ Hufnagel JC, Hofmann T (2008). "Orosensory-directed identification of astringent mouthfeel and bitter-tasting compounds in red wine". J Agric Food Chem. 56 (4): 1376–1386. doi:10.1021/jf073031n. PMID 18193832.
- ^ Owen, R. W.; Haubner, R.; Hull, W. E.; Erben, G.; Spiegelhalder, B.; Bartsch, H.; Haber, B. (2003). "Isolation and structure elucidation of the major individual polyphenols in carob fibre". Food and Chemical Toxicology. 41 (12): 1727–1738. doi:10.1016/S0278-6915(03)00200-X. PMID 14563398.
- ^ Polyphenol extraction from foods. Maria teresa Escribano-Bailon and Celestino Santos-Buelga
- ^ Pan, X (2003). "Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves". Chemical Engineering and Processing. 42 (2): 129–33. doi:10.1016/S0255-2701(02)00037-5.
- ^ Palma, M; Taylor, L (1999). "Extraction of polyphenolic compounds from grape seeds with near critical carbon dioxide". Journal of Chromatography A. 849 (1): 117–24. doi:10.1016/S0021-9673(99)00569-5. PMID 10444839.
- ^ Alonsosalces, R; Korta, E; Barranco, A; Berrueta, L; Gallo, B; Vicente, F (2001). "Pressurized liquid extraction for the determination of polyphenols in apple". Journal of Chromatography A. 933 (1–2): 37–43. doi:10.1016/S0021-9673(01)01212-2. PMID 11758745.
- ^ Sineiro, J.; Domínguez, H.; Núñez, M. J.; Lema, J. M. (1996). "Ethanol extraction of polyphenols in an immersion extractor. Effect of pulsing flow". Journal of the American Oil Chemists' Society. 73 (9): 1121–5. doi:10.1007/BF02523372.
- ^ Arranz, Sara; Saura-Calixto, Fulgencio; Shaha, Shika; Kroon, Paul A. (2009). "High Contents of Nonextractable Polyphenols in Fruits Suggest That Polyphenol Contents of Plant Foods Have Been Underestimated". Journal of Agricultural and Food Chemistry. 57 (16): 7298–303. doi:10.1021/jf9016652. PMID 19637929.
- ^ Nawaz, H; Shi, J; Mittal, G; Kakuda, Y (2006). "Extraction of polyphenols from grape seeds and concentration by ultrafiltration". Separation and Purification Technology. 48 (2): 176–81. doi:10.1016/j.seppur.2005.07.006.
- ^ Tempel, A. S. (1982). "Tannin-measuring techniques". Journal of Chemical Ecology. 8 (10): 1289–1298. doi:10.1007/BF00987762.
- ^ Grossi, Marco; Di Lecce, Giuseppe; Arru, Marco; Gallina Toschi, Tullia; Riccò, Bruno (2015). "An opto-electronic system for in-situ determination of peroxide value and total phenol content in olive oil". Journal of Food Engineering. 146: 1–7. doi:10.1016/j.jfoodeng.2014.08.015.
- ^ Gani, M.; McGuinness, B. J.; Da Vies, A. P. (1998). "Monoclonal antibodies against tea polyphenols: A novel immunoassay to detect polyphenols in biological fluids". Food and Agricultural Immunology. 10: 13–22. doi:10.1080/09540109809354964.
- ^ Walker, Richard B.; Everette, Jace D. (2009). "Comparative Reaction Rates of Various Antioxidants with ABTS Radical Cation". Journal of Agricultural and Food Chemistry. 57 (4): 1156–61. doi:10.1021/jf8026765. PMID 19199590.
- ^ Roy, M. K.; Koide, M.; Rao, T. P.; Okubo, T.; Ogasawara, Y.; Juneja, L. R. (2010). "ORAC and DPPH assay comparison to assess antioxidant capacity of tea infusions: Relationship between total polyphenol and individual catechin content". International Journal of Food Sciences and Nutrition. 61 (2): 109–124. doi:10.3109/09637480903292601. PMID 20109129.
- ^ Pulido, R.; Bravo, L.; Saura-Calixto, F. (2000). "Antioxidant Activity of Dietary Polyphenols As Determined by a Modified Ferric Reducing/Antioxidant Power Assay". Journal of Agricultural and Food Chemistry. 48 (8): 3396–3402. doi:10.1021/jf9913458. PMID 10956123.
- ^ Meyer, A. S.; Yi, O. S.; Pearson, D. A.; Waterhouse, A. L.; Frankel, E. N. (1997). "Inhibition of Human Low-Density Lipoprotein Oxidation in Relation to Composition of Phenolic Antioxidants in Grapes (Vitis vinifera)". Journal of Agricultural and Food Chemistry. 45 (5): 1638–1643. doi:10.1021/jf960721a.
- ^ Mello, L; Sotomayor, Maria Del Pilar Taboada; Kubota, Lauro Tatsuo (2003). "HRP-based amperometric biosensor for the polyphenols determination in vegetables extract". Sensors and Actuators B: Chemical. 96 (3): 636–45. doi:10.1016/j.snb.2003.07.008.
External links
- Phenol-Explorer Electronic database on polyphenol content in foods
