Helicase
Helicases are a class of enzymes thought to be vital to all organisms. Their main function is to unpack an organism's genetic material. Helicases are motor proteins that move directionally along a nucleic acid phosphodiester backbone, separating two hybridized nucleic acid strands (hence helic- + -ase), using energy from ATP hydrolysis. There are many helicases, representing the great variety of processes in which strand separation must be catalyzed. Approximately 1% of eukaryotic genes code for helicases.[1]
The human genome codes for 95 non-redundant helicases: 64 RNA helicases and 31 DNA helicases.[2] Many cellular processes, such as DNA replication, transcription, translation, recombination, DNA repair, and ribosome biogenesis involve the separation of nucleic acid strands that necessitates the use of helicases. Some specialized helicases are also involved in sensing of viral nucleic acids during infection and fulfill a immunological function.A helicase is an enzyme that plays a crucial role in the DNA replication and repair processes. Its primary function is to unwind the double-stranded DNA molecule by breaking the hydrogen bonds between the complementary base pairs, allowing the DNA strands to separate. This creates a replication fork, which serves as a template for synthesizing new DNA strands. Helicase is an essential component of cellular mechanisms that ensures accurate DNA replication and maintenance of genetic information. DNA helicase catalyzes regression. RecG and the enzyme PriA work together to rewind duplex DNA, creating a Holliday junction. RecG releases bound proteins and the PriA helicase facilitates DNA reloading to resume DNA replication. RecG replaces the single-strand binding protein (SSB), which regulates the helicase-fork loading sites during fork regression. The SSB protein interacts with DNA helicases PriA and RecG to recover stalled DNA replication forks. These enzymes must bind to the SSB-helicase to be loaded onto stalled forks. Thermal sliding and DNA duplex binding are possibly supported by the wedge domain of RecG's association with the SSB linker. In a regression reaction facilitated by RecG and ATPHollidayjunctions are created for later processing.
Function[edit]
Helicases are often used to separate strands of a DNA double helix or a self-annealed RNA molecule using the energy from ATP hydrolysis, a process characterized by the breaking of hydrogen bonds between annealed nucleotide bases. They also function to remove nucleic acid-associated proteins and catalyze homologous DNA recombination.[3] Metabolic processes of RNA such as translation, transcription, ribosome biogenesis, RNA splicing, RNA transport, RNA editing, and RNA degradation are all facilitated by helicases.[3] Helicases move incrementally along one nucleic acid strand of the duplex with a directionality and processivity specific to each particular enzyme.
Helicases adopt different structures and oligomerization states. Whereas DnaB-like helicases unwind DNA as ring-shaped hexamers, other enzymes have been shown to be active as monomers or dimers. Studies have shown that helicases may act passively, waiting for uncatalyzed unwinding to take place and then translocating between displaced strands,[4] or can play an active role in catalyzing strand separation using the energy generated in ATP hydrolysis.[5] In the latter case, the helicase acts comparably to an active motor, unwinding and translocating along its substrate as a direct result of its ATPase activity.[6] Helicases may process much faster in vivo than in vitro due to the presence of accessory proteins that aid in the destabilization of the fork junction.[6]

Activation barrier in helicase activity[edit]
Enzymatic helicase action, such as unwinding nucleic acids is achieved through the lowering of the activation barrier () of each specific action.[7][5][8][9] The activation barrier is a result of various factors, and can be defined using the following equation, where
= number of unwound base pairs (bps),
= free energy of base pair formation,
= reduction of free energy due to helicase, and
= reduction of free energy due to unzipping forces.
Factors that contribute to the height of the activation barrier include: specific nucleic acid sequence of the molecule involved, the number of base pairs involved, tension present on the replication fork, and destabilization forces.[7][5][8][9]
Active and passive helicases[edit]
The size of the activation barrier to overcome by the helicase contributes to its classification as an active or passive helicase. In passive helicases, a significant activation barrier exists (defined as , where is Boltzmann's constant and is temperature of the system). Due to this significant activation barrier, its unwinding progression is affected largely by the sequence of nucleic acids within the molecule to unwind, and the presence of destabilization forces acting on the replication fork.[7][5][8][9] Certain nucleic acid combinations will decrease unwinding rates (i.e. guanine and cytosine), while various destabilizing forces can increase the unwinding rate.[5][8][9] In passive systems, the rate of unwinding () is less than the rate of translocation () (translocation along the single-strand nucleic acid, ssNA), due to its reliance on the transient unraveling of the base pairs at the replication fork to determine its rate of unwinding.[7][5][8][9]
In active helicases, , where the system lacks a significant barrier, as the helicase can destabilize the nucleic acids, unwinding the double-helix at a constant rate, regardless of the nucleic acid sequence. In active helicases, is closer to , due to the active helicase ability to directly destabilize the replication fork to promote unwinding.[7][5][8][9]
Active helicases show similar behavior when acting on both double-strand nucleic acids, dsNA, or ssNA, in regards to the rates of unwinding and rates of translocation, where in both systems and are approximately equal.
These two categories of helicases may also be modeled as mechanisms. In such models, the passive helicases are conceptualized as Brownian ratchets, driven by thermal fluctuations and subsequent anisotropic gradients across the DNA lattice. The active helicases, in contrast, are conceptualized as stepping motors – also known as powerstroke motors – utilizing either a conformational "inch worm" or a hand-over-hand "walking" mechanism to progress.[10] Depending upon the organism, such helix-traversing progress can occur at rotational speeds in the range of 5,000 [11] to 10,000 [12] R.P.M.
History of DNA helicases[edit]
DNA helicases were discovered in E. coli in 1976. This helicase was described as a "DNA unwinding enzyme" that is "found to denature DNA duplexes in an ATP-dependent reaction, without detectably degrading".[13] The first eukaryotic DNA helicase discovered was in 1978 in the lily plant.[14] Since then, DNA helicases were discovered and isolated in other bacteria, viruses, yeast, flies, and higher eukaryotes.[15] To date, at least 14 different helicases have been isolated from single celled organisms, 6 helicases from bacteriophages, 12 from viruses, 15 from yeast, 8 from plants, 11 from calf thymus, and approximately 25 helicases from human cells.[16] Below is a history of helicase discovery:
- 1976 – Discovery and isolation of E. coli-based DNA helicase[13]
- 1978 – Discovery of the first eukaryotic DNA helicases, isolated from the lily plant[14]
- 1982 – "T4 gene 41 protein" is the first reported bacteriophage DNA helicase[15]
- 1985 – First mammalian DNA helicases isolated from calf thymus[17]
- 1986 – SV40 large tumor antigen reported as a viral helicase (1st reported viral protein that was determined to serve as a DNA helicase)[18]
- 1986 – ATPaseIII, a yeast protein, determined to be a DNA helicase[19]
- 1988 – Discovery of seven conserved amino acid domains determined to be helicase motifs
- 1989 – Designation of DNA helicase Superfamily I and Superfamily II[20]
- 1989 – Identification of the DEAD box helicase family[21]
- 1990 – Isolation of a human DNA helicase[22]
- 1992 – Isolation of the first reported mitochondrial DNA helicase (from bovine brain)[23]
- 1996 – Report of the discovery of the first purified chloroplast DNA helicase from the pea[24]
- 2002 – Isolation and characterization of the first biochemically active malarial parasite DNA helicase – Plasmodium cynomolgi.[25]
Structural features[edit]
The common function of helicases accounts for the fact that they display a certain degree of amino acid sequence homology; they all possess sequence motifs located in the interior of their primary structure, involved in ATP binding, ATP hydrolysis and translocation along the nucleic acid substrate. The variable portion of the amino acid sequence is related to the specific features of each helicase.
The presence of these helicase motifs allows putative helicase activity to be attributed to a given protein, but does not necessarily confirm it as an active helicase. Conserved motifs do, however, support an evolutionary homology among enzymes. Based on these helicase motifs, a number of helicase superfamilies have been distinguished.
Superfamilies[edit]
Helicases are classified in 6 groups (superfamilies) based on their shared sequence motifs.[26] Helicases not forming a ring structure are in superfamilies 1 and 2, and ring-forming helicases form part of superfamilies 3 to 6.[27] Helicases are also classified as α or β depending on if they work with single or double-strand DNA; α helicases work with single-strand DNA and β helicases work with double-strand DNA. They are also classified by translocation polarity. If translocation occurs 3’-5’ the helicase is type A; if translocation occurs 5’-3’ it is type B.[26]
- Superfamily 1 (SF1): This superfamily can be further subdivided into SF1A and SF1B helicases.[26] In this group helicases can have either 3’-5’ (SF1A subfamily) or 5’-3’(SF1B subfamily) translocation polarity.[26][28] The most known SF1A helicases are Rep and UvrD in gram-negative bacteria and PcrA helicase from gram-positive bacteria.[26] The most known Helicases in the SF1B group are RecD and Dda helicases.[26] They have a RecA-like-fold core.[27]
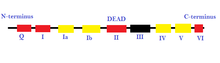
- Superfamily 2 (SF2): This is the largest group of helicases that are involved in varied cellular processes.[26][2] They are characterized by the presence of nine conserved motifs: Q, I, Ia, Ib, and II through VI.[2] This group is mainly composed of DEAD-box RNA helicases.[27] Some other helicases included in SF2 are the RecQ-like family and the Snf2-like enzymes.[26] Most of the SF2 helicases are type A with a few exceptions such as the XPD family.[26] They have a RecA-like-fold core.[27]
- Superfamily 3 (SF3): Superfamily 3 consists of AAA+ helicases encoded mainly by small DNA viruses and some large nucleocytoplasmic DNA viruses.[29][30] They have a 3’-5’ translocation directionality, meaning that they are all type A helicases.[26] The most known SF3 helicase is the papilloma virus E1 helicase.[26]
- Superfamily 4 (SF4): All SF4 family helicases have a type B polarity (5’-3’). They have a RecA fold.[26] The most studied SF4 helicase is gp4 from bacteriophage T7.[26]
- Superfamily 5 (SF5): Rho proteins conform the SF5 group. They have a RecA fold.[26]
- Superfamily 6 (SF6): They contain the core AAA+ that is not included in the SF3 classification.[26] Some proteins in the SF6 group are: mini chromosome maintenance MCM, RuvB, RuvA, and RuvC.[26]
All helicases are members of a P-loop, or Walker motif-containing family.
Helicase disorders and diseases[edit]
ATRX helicase mutations[edit]
The ATRX gene encodes the ATP-dependent helicase, ATRX (also known as XH2 and XNP) of the SNF2 subgroup family, that is thought to be responsible for functions such as chromatin remodeling, gene regulation, and DNA methylation.[31][32][33][34] These functions assist in prevention of apoptosis, resulting in cortical size regulation, as well as a contribution to the survival of hippocampal and cortical structures, affecting memory and learning.[31] This helicase is located on the X chromosome (Xq13.1-q21.1), in the pericentromeric heterochromatin and binds to heterochromatin protein 1.[31][33] Studies have shown that ATRX plays a role in rDNA methylation and is essential for embryonic development.[35] Mutations have been found throughout the ATRX protein, with over 90% of them being located in the zinc finger and helicase domains.[36] Mutations of ATRX can result in X-linked-alpha-thalassaemia-mental retardation (ATR-X syndrome).[31]
Various types of mutations found in ATRX have been found to be associated with ATR-X, including most commonly single-base missense mutations, as well as nonsense, frameshift, and deletion mutations.[34] Characteristics of ATR-X include: microcephaly, skeletal and facial abnormalities, mental retardation, genital abnormalities, seizures, limited language use and ability, and alpha-thalassemia.[31][35][32] The phenotype seen in ATR-X suggests that the mutation of ATRX gene causes the downregulation of gene expression, such as the alpha-globin genes.[32] It is still unknown what causes the expression of the various characteristics of ATR-X in different patients.[35]
XPD helicase point mutations[edit]
XPD (Xeroderma pigmentosum factor D, also known as protein ERCC2) is a 5'-3', Superfamily II, ATP-dependent helicase containing iron-sulphur cluster domains.[26][37] Inherited point mutations in XPD helicase have been shown to be associated with accelerated aging disorders such as Cockayne syndrome (CS) and trichothiodystrophy (TTD).[38] Cockayne syndrome and trichothiodystrophy are both developmental disorders involving sensitivity to UV light and premature aging, and Cockayne syndrome exhibits severe mental retardation from the time of birth.[38] The XPD helicase mutation has also been implicated in xeroderma pigmentosum (XP), a disorder characterized by sensitivity to UV light and resulting in a several 1000-fold increase in the development of skin cancer.[38]
XPD is an essential component of the TFIIH complex, a transcription and repair factor in the cell.[38][39][40][41][42] As part of this complex, it facilitates nucleotide excision repair by unwinding DNA.[38] TFIIH assists in repairing damaged DNA such as sun damage.[38][39][40][41][42] A mutation in the XPD helicase that helps form this complex and contributes to its function causes the sensitivity to sunlight seen in all three diseases, as well as the increased risk of cancer seen in XP and premature aging seen in trichothiodystrophy and Cockayne syndrome.[38]
XPD helicase mutations leading to trichothiodystrophy are found throughout the protein in various locations involved in protein-protein interactions.[38] This mutation results in an unstable protein due to its inability to form stabilizing interactions with other proteins at the points of mutations.[38] This, in turn, destabilizes the entire TFIIH complex, which leads to defects with transcription and repair mechanisms of the cell.[38]
It has been suggested that XPD helicase mutations leading to Cockayne syndrome could be the result of mutations within XPD, causing rigidity of the protein and subsequent inability to switch from repair functions to transcription functions due to a "locking" in repair mode.[38] This could cause the helicase to cut DNA segments meant for transcription.[38] Although current evidence points to a defect in the XPD helicase resulting in a loss of flexibility in the protein in cases of Cockayne syndrome, it is still unclear how this protein structure leads to the symptoms described in Cockayne syndrome.[38]
In xeroderma pigmentosa, the XPD helicase mutation exists at the site of ATP or DNA binding.[38] This results in a structurally functional helicase able to facilitate transcription, however it inhibits its function in unwinding DNA and DNA repair.[38] The lack of a cell's ability to repair mutations, such as those caused by sun damage, is the cause of the high cancer rate in xeroderma pigmentosa patients.
RecQ family mutations[edit]

RecQ helicases (3'-5') belong to the Superfamily II group of helicases, which help to maintain stability of the genome and suppress inappropriate recombination.[43][44] Deficiencies and/or mutations in RecQ family helicases display aberrant genetic recombination and/or DNA replication, which leads to chromosomal instability and an overall decreased ability to proliferate.[43] Mutations in RecQ family helicases BLM, RECQL4, and WRN, which play a role in regulating homologous recombination, have been shown to result in the autosomal recessive diseases Bloom syndrome (BS), Rothmund–Thomson syndrome (RTS), and Werner syndrome (WS), respectively.[44][45]
Bloom syndrome is characterized by a predisposition to cancer with early onset, with a mean age-of-onset of 24 years.[44][46] Cells of Bloom syndrome patients show a high frequency of reciprocal exchange between sister chromatids (SCEs) and excessive chromosomal damage.[47] There is evidence to suggest that BLM plays a role in rescuing disrupted DNA replication at replication forks.[47]
Werner syndrome is a disorder of premature aging, with symptoms including early onset of atherosclerosis and osteoporosis and other age related diseases, a high occurrence of sarcoma, and death often occurring from myocardial infarction or cancer in the 4th to 6th decade of life.[44][48] Cells of Werner syndrome patients exhibit a reduced reproductive lifespan with chromosomal breaks and translocations, as well as large deletions of chromosomal components, causing genomic instability.[48]
Rothmund-Thomson syndrome, also known as poikiloderma congenitale, is characterized by premature aging, skin and skeletal abnormalities, rash, poikiloderma, juvenile cataracts, and a predisposition to cancers such as osteosarcomas.[44][49] Chromosomal rearrangements causing genomic instability are found in the cells of Rothmund-Thomson syndrome patients. RecQ is a family of DNA helicase enzymes that are found in various organisms including bacteria, archaea, and eukaryotes (like humans). These enzymes play important roles in DNA metabolism during DNA replication, recombination, and repair. There are five known RecQ helicase proteins in humans: RecQ1, BLM, WRN, RecQ4, and RecQ5. Mutations in some of these genes are associated with genetic disorders. For instance, mutations in the BLM gene cause Bloom syndrome, which is characterized by increased cancer risk and other health issues.[50] Mutations in the WRN gene lead to Werner syndrome, a condition characterized by premature aging and an increased risk of age-related diseases. RecQ helicases are crucial for maintaining genomic stability and integrity. They help prevent the accumulation of genetic abnormalities that can lead to diseases like cancer. Genome integrity depends on the RecQ DNA helicase family, which includes DNA repair, recombination, replication, and transcription processes. Genome instability and early aging are conditions that arise from mutations in human RecQ helicases.[51] RecQ helicase Sgs1 is missing in yeast cells, making them useful models for comprehending human cell abnormalities and the RecQ helicase function.[52] The RecQ helicase family member, RECQ1, is connected to a small number of uncommon genetic cancer disorders in individuals. It participates in transcription, the cell cycle, and DNA repair. According to recent research, missense mutations in the RECQ1 gene may play a role in the development of familial breast cancer. DNA helicases are frequently attracted to regions of DNA damage and are essential for cellular DNA replication, recombination, repair, and transcription. Chemical manipulation of their molecular processes can change the rate at which cancer cells divide, as well as, the efficiency of transactions and cellular homeostasis. Small-molecule-induced entrapment of DNA helicases, a type of DNA metabolic protein, may have deleterious consequences on rapidly proliferating cancer cells, which could be effective in cancer treatment.
During meiosis DNA double-strand breaks and other DNA damages in a chromatid are repaired by homologous recombination using either the sister chromatid or a homologous non-sister chromatid as template. This repair can result in a crossover (CO) or, more frequently, a non-crossover (NCO) recombinant. In the yeast Schizosaccharomyces pombe the FANCM-family DNA helicase FmI1 directs NCO recombination formation during meiosis.[53] The RecQ-type helicase Rqh1 also directs NCO meiotic recombination.[54] These helicases, through their ability to unwind D-loop intermediates, promote NCO recombination by the process of synthesis-dependent strand annealing.
In the plant Arabidopsis thaliana, FANCM helicase promotes NCO and antagonizes the formation of CO recombinants.[55] Another helicase, RECQ4A/B, also independently reduces COs. It was suggested that COs are restricted because of the long term costs of CO recombination, that is, the breaking up of favorable genetic combinations of alleles built up by past natural selection.[55]
RNA helicases[edit]

RNA helicases are essential for most processes of RNA metabolism such as ribosome biogenesis, pre-mRNA splicing, and translation initiation. They also play an important role in sensing viral RNAs.[56] RNA helicases are involved in the mediation of antiviral immune response because they can identify foreign RNAs in vertebrates. About 80% of all viruses are RNA viruses and they contain their own RNA helicases.[57] Defective RNA helicases have been linked to cancers, infectious diseases and neuro-degenerative disorders.[56] Some neurological disorders associated with defective RNA helicases are: amyotrophic lateral sclerosis, spinal muscular atrophy, spinocerebellar ataxia type-2, Alzheimer disease, and lethal congenital contracture syndrome.[57]
RNA helicases and DNA helicases can be found together in all the helicase superfamilies except for SF6.[58][59] All the eukaryotic RNA helicases that have been identified up to date are non-ring forming and are part of SF1 and SF2. On the other hand, ring-forming RNA helicases have been found in bacteria and viruses.[56] However, not all RNA helicases exhibit helicase activity as defined by enzymatic function, i.e., proteins of the Swi/Snf family. Although these proteins carry the typical helicase motifs, hydrolize ATP in a nucleic acid-dependent manner, and are built around a helicase core, in general, no unwinding activity is observed.[60]
RNA helicases that do exhibit unwinding activity have been characterized by at least two different mechanisms: canonical duplex unwinding and local strand separation. Canonical duplex unwinding is the stepwise directional separation of a duplex strand, as described above, for DNA unwinding. However, local strand separation occurs by a process wherein the helicase enzyme is loaded at any place along the duplex. This is usually aided by a single-strand region of the RNA, and the loading of the enzyme is accompanied with ATP binding.[61] Once the helicase and ATP are bound, local strand separation occurs, which requires binding of ATP but not the actual process of ATP hydrolysis.[62] Presented with fewer base pairs the duplex then dissociates without further assistance from the enzyme. This mode of unwinding is used by the DEAD/DEAH box helicases.[63]
An RNA helicase database[64] is currently available online that contains a comprehensive list of RNA helicases with information such as sequence, structure, and biochemical and cellular functions.[56]
Diagnostic tools for helicase measurement[edit]
Measuring and monitoring helicase activity[edit]
Various methods are used to measure helicase activity in vitro. These methods range from assays that are qualitative (assays that usually entail results that do not involve values or measurements) to quantitative (assays with numerical results that can be utilized in statistical and numerical analysis). In 1982–1983, the first direct biochemical assay was developed for measuring helicase activity.[15][65] This method was called a "strand displacement assay".
- Strand displacement assay involves the radiolabeling of DNA duplexes. Following helicase treatment, the single-strand DNA is visually detected as separate from the double-strand DNA by non-denaturing PAGE electrophoresis. Following detection of the single-strand DNA, the amount of radioactive tag that is on the single-strand DNA is quantified to give a numerical value for the amount of double-strand DNA unwinding.
- The strand displacement assay is acceptable for qualitative analysis, its inability to display results for more than a single time point, its time consumption, and its dependence on radioactive compounds for labeling warranted the need for development of diagnostics that can monitor helicase activity in real time.
Other methods were later developed that incorporated some, if not all of the following: high-throughput mechanics, the use of non-radioactive nucleotide labeling, faster reaction time/less time consumption, real-time monitoring of helicase activity (using kinetic measurement instead of endpoint/single point analysis). These methodologies include: "a rapid quench flow method, fluorescence-based assays, filtration assays, a scintillation proximity assay, a time resolved fluorescence resonance energy transfer assay, an assay based on flashplate technology, homogenous time-resolved fluorescence quenching assays, and electrochemiluminescence-based helicase assays".[16] With the use of specialized mathematical equations, some of these assays can be utilized to determine how many base paired nucleotides a helicase can break per hydrolysis of 1 ATP molecule.[66]
Commercially available diagnostic kits are also available. One such kit is the "Trupoint" diagnostic assay from PerkinElmer, Inc. This assay is a time-resolved fluorescence quenching assay that utilizes the PerkinElmer "SignalClimb" technology that is based on two labels that bind in close proximity to one another but on opposite DNA strands. One label is a fluorescent lanthanide chelate, which serves as the label that is monitored through an adequate 96/384 well plate reader. The other label is an organic quencher molecule. The basis of this assay is the "quenching" or repressing of the lanthanide chelate signal by the organic quencher molecule when the two are in close proximity – as they would be when the DNA duplex is in its native state. Upon helicase activity on the duplex, the quencher and lanthanide labels get separated as the DNA is unwound. This loss in proximity negates the quenchers ability to repress the lanthanide signal, causing a detectable increase in fluorescence that is representative of the amount of unwound DNA and can be used as a quantifiable measurement of helicase activity. The execution and use of single-molecule fluorescence imaging techniques, focusing on methods that include optical trapping in conjunction with epifluorescent imaging, and also surface immobilization in conjunction with total internal reflection fluorescence visualization. Combined with microchannel flow cells and microfluidic control, allow individual fluorescently labeled protein and DNA molecules to be imaged and tracked, affording measurement of DNA unwinding and translocation at single-molecule resolution.[67]
Determining helicase polarity[edit]
Helicase polarity, which is also deemed "directionality", is defined as the direction (characterized as 5'→3' or 3'→5') of helicase movement on the DNA/RNA single-strand along which it is moving. This determination of polarity is vital in f.ex. determining whether the tested helicase attaches to the DNA leading strand, or the DNA lagging strand. To characterize this helicase feature, a partially duplex DNA is used as the substrate that has a central single-strand DNA region with different lengths of duplex regions of DNA (one short region that runs 5'→3' and one longer region that runs 3'→5') on both sides of this region.[68] Once the helicase is added to that central single-strand region, the polarity is determined by characterization on the newly formed single-strand DNA.
See also[edit]
- Chromodomain helicase DNA binding protein: CHD1, CHD1L, CHD2, CHD3, CHD4, CHD5, CHD6, CHD7, CHD8, CHD9
- DEAD box/DEAD/DEAH box helicase: DDX3X, DDX5, DDX6, DDX10, DDX11, DDX12, DDX58, DHX8, DHX9, DHX37, DHX40, DHX58
- ASCC3, BLM, BRIP1, DNA2, FBXO18, FBXO30, HELB, HELLS, HELQ, HELZ, HFM1, HLTF, IFIH1, NAV2, PIF1, RECQL, RTEL1, SHPRH, SMARCA4, SMARCAL1, WRN, WRNIP1
- RNA helicase database
References[edit]
- ^ Wu Y (2012). "Unwinding and rewinding: double faces of helicase?". Journal of Nucleic Acids. 2012: 140601. doi:10.1155/2012/140601. PMC 3409536. PMID 22888405.
- ^ a b c Umate P, Tuteja N, Tuteja R (January 2011). "Genome-wide comprehensive analysis of human helicases". Communicative & Integrative Biology. 4 (1): 118–137. doi:10.4161/cib.13844. PMC 3073292. PMID 21509200.
- ^ a b Patel SS, Donmez I (July 2006). "Mechanisms of helicases". The Journal of Biological Chemistry. 281 (27): 18265–18268. doi:10.1074/jbc.R600008200. PMID 16670085.
- ^ Lionnet T, Spiering MM, Benkovic SJ, Bensimon D, Croquette V (December 2007). "Real-time observation of bacteriophage T4 gp41 helicase reveals an unwinding mechanism". Proceedings of the National Academy of Sciences of the United States of America. 104 (50): 19790–19795. Bibcode:2007PNAS..10419790L. doi:10.1073/pnas.0709793104. PMC 2148377. PMID 18077411.
- ^ a b c d e f g Johnson DS, Bai L, Smith BY, Patel SS, Wang MD (June 2007). "Single-molecule studies reveal dynamics of DNA unwinding by the ring-shaped T7 helicase". Cell. 129 (7): 1299–1309. doi:10.1016/j.cell.2007.04.038. PMC 2699903. PMID 17604719.
- ^ a b "Researchers solve mystery of how DNA strands separate". 2007-07-03. Retrieved 2007-07-05.
- ^ a b c d e Betterton MD, Jülicher F (2005-08-31). "Erratum: Opening of nucleic-acid double strands by helicases: Active versus passive opening [Phys. Rev. E 71, 011904 (2005)]". Physical Review E. 72 (2): 029906. Bibcode:2005PhRvE..72b9906B. doi:10.1103/PhysRevE.72.029906.
- ^ a b c d e f Manosas M, Xi XG, Bensimon D, Croquette V (September 2010). "Active and passive mechanisms of helicases". Nucleic Acids Research. 38 (16): 5518–5526. doi:10.1093/nar/gkq273. PMC 2938219. PMID 20423906.
- ^ a b c d e f Jarillo J, Ibarra B, Cao-García FJ (2021). "DNA replication: In vitro single-molecule manipulation data analysis and models". Computational and Structural Biotechnology Journal. 19: 3765–3778. doi:10.1016/j.csbj.2021.06.032. PMC 8267548. PMID 34285777.
- ^ Wu, C. G. and Spies, M.: Overview: What are Helicases? In: Spies, M. (Ed.): [1]. Springer Science+Business Media, NY, 2013
- ^ "Kevin Ahern's Biochemistry (BB 451/551) at Oregon State University". oregonstate.edu. Archived from the original on 2021-01-26. Retrieved 2024-01-03.
- ^ 3-D Animation Library; Replication: [2] (Advanced)
- ^ a b Abdel-Monem M, Dürwald H, Hoffmann-Berling H (June 1976). "Enzymic unwinding of DNA. 2. Chain separation by an ATP-dependent DNA unwinding enzyme". European Journal of Biochemistry. 65 (2): 441–449. doi:10.1111/j.1432-1033.1976.tb10359.x. PMID 133023.
- ^ a b Hotta Y, Stern H (May 1978). "DNA unwinding protein from meiotic cells of Lilium". Biochemistry. 17 (10): 1872–1880. doi:10.1021/bi00603a011. PMID 207302.
- ^ a b c Venkatesan M, Silver LL, Nossal NG (October 1982). "Bacteriophage T4 gene 41 protein, required for the synthesis of RNA primers, is also a DNA helicase". The Journal of Biological Chemistry. 257 (20): 12426–12434. doi:10.1016/S0021-9258(18)33731-1. PMID 6288720.
- ^ a b Tuteja N, Tuteja R (May 2004). "Prokaryotic and eukaryotic DNA helicases. Essential molecular motor proteins for cellular machinery". European Journal of Biochemistry. 271 (10): 1835–1848. doi:10.1111/j.1432-1033.2004.04093.x. PMC 7164108. PMID 15128294.
- ^ Hübscher U, Stalder HP (August 1985). "Mammalian DNA helicase". Nucleic Acids Research. 13 (15): 5471–5483. doi:10.1093/nar/13.15.5471. PMC 321884. PMID 3162158.
- ^ Stahl H, Dröge P, Knippers R (August 1986). "DNA helicase activity of SV40 large tumor antigen". The EMBO Journal. 5 (8): 1939–1944. doi:10.1002/j.1460-2075.1986.tb04447.x. PMC 1167061. PMID 3019672.
- ^ Sugino A, Ryu BH, Sugino T, Naumovski L, Friedberg EC (September 1986). "A new DNA-dependent ATPase which stimulates yeast DNA polymerase I and has DNA-unwinding activity". The Journal of Biological Chemistry. 261 (25): 11744–11750. doi:10.1016/S0021-9258(18)67306-5. PMID 3017945.
- ^ Gorbalenya AE, Koonin EV, Donchenko AP, Blinov VM (June 1989). "Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes". Nucleic Acids Research. 17 (12): 4713–4730. doi:10.1093/nar/17.12.4713. PMC 318027. PMID 2546125.
- ^ Linder, P., Lasko, P.F., Ashburner, M., Leroy, P., Nielson, P.J., Nishi, K., Schneir, J., Slonimski, P.P. (1989) Birth of the DEAD-box. Nature (London) 337, 121-122.
- ^ Tuteja N, Tuteja R, Rahman K, Kang LY, Falaschi A (December 1990). "A DNA helicase from human cells". Nucleic Acids Research. 18 (23): 6785–6792. doi:10.1093/nar/18.23.6785. PMC 332732. PMID 1702201.
- ^ Hehman GL, Hauswirth WW (September 1992). "DNA helicase from mammalian mitochondria". Proceedings of the National Academy of Sciences of the United States of America. 89 (18): 8562–8566. Bibcode:1992PNAS...89.8562H. doi:10.1073/pnas.89.18.8562. PMC 49960. PMID 1326759.
- ^ Tuteja N, Phan TN, Tewari KK (May 1996). "Purification and characterization of a DNA helicase from pea chloroplast that translocates in the 3'-to-5' direction". European Journal of Biochemistry. 238 (1): 54–63. doi:10.1111/j.1432-1033.1996.0054q.x. PMID 8665952.
- ^ Tuteja R, Malhotra P, Song P, Tuteja N, Chauhan VS (2002). "Isolation and characterization of an eIF-4A homologue from Plasmodium cynomolgi". Molecular and Biochemical Parasitology. 124 (1–2): 79–83. doi:10.1016/S0166-6851(02)00205-0. PMID 12387853.
- ^ a b c d e f g h i j k l m n o p q Singleton MR, Dillingham MS, Wigley DB (2007). "Structure and mechanism of helicases and nucleic acid translocases". Annual Review of Biochemistry. 76: 23–50. doi:10.1146/annurev.biochem.76.052305.115300. PMID 17506634.
- ^ a b c d Fairman-Williams ME, Guenther UP, Jankowsky E (June 2010). "SF1 and SF2 helicases: family matters". Current Opinion in Structural Biology. 20 (3): 313–324. doi:10.1016/j.sbi.2010.03.011. PMC 2916977. PMID 20456941.
- ^ Stelter M, Acajjaoui S, McSweeney S, Timmins J (2013). "Structural and mechanistic insight into DNA unwinding by Deinococcus radiodurans UvrD". PLOS ONE. 8 (10): e77364. Bibcode:2013PLoSO...877364S. doi:10.1371/journal.pone.0077364. PMC 3797037. PMID 24143224.
- ^ Iyer LM, Aravind L, Koonin EV (December 2001). "Common origin of four diverse families of large eukaryotic DNA viruses". Journal of Virology. 75 (23): 11720–11734. doi:10.1128/JVI.75.23.11720-11734.2001. PMC 114758. PMID 11689653.
- ^ Iyer LM, Leipe DD, Koonin EV, Aravind L (2004). "Evolutionary history and higher order classification of AAA+ ATPases". Journal of Structural Biology. 146 (1–2): 11–31. doi:10.1016/j.jsb.2003.10.010. PMID 15037234.
- ^ a b c d e Ropers HH, Hamel BC (January 2005). "X-linked mental retardation". Nature Reviews. Genetics. 6 (1): 46–57. doi:10.1038/nrg1501. PMID 15630421. S2CID 427210.
- ^ a b c Gibbons RJ, Picketts DJ, Villard L, Higgs DR (March 1995). "Mutations in a putative global transcriptional regulator cause X-linked mental retardation with alpha-thalassemia (ATR-X syndrome)". Cell. 80 (6): 837–845. doi:10.1016/0092-8674(95)90287-2. PMID 7697714.
- ^ a b Nextprot Online Protein Database. " ATRX-Transcriptional regulator ATRX.", Retrieved on 12 November 2012.
- ^ a b Picketts DJ, Higgs DR, Bachoo S, Blake DJ, Quarrell OW, Gibbons RJ (December 1996). "ATRX encodes a novel member of the SNF2 family of proteins: mutations point to a common mechanism underlying the ATR-X syndrome". Human Molecular Genetics. 5 (12): 1899–1907. doi:10.1093/hmg/5.12.1899. PMID 8968741.
- ^ a b c Gibbons R (May 2006). "Alpha thalassaemia-mental retardation, X linked". Orphanet Journal of Rare Diseases. 1: 15. doi:10.1186/1750-1172-1-15. PMC 1464382. PMID 16722615.
- ^ Stevenson RE (1993). Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, Gripp KW, Amemiya A (eds.). "Alpha-Thalassemia X-Linked Intellectual Disability Syndrome". GeneReviews. Seattle (WA): University of Washington, Seattle. PMID 20301622.
- ^ Rudolf J, Rouillon C, Schwarz-Linek U, White MF (January 2010). "The helicase XPD unwinds bubble structures and is not stalled by DNA lesions removed by the nucleotide excision repair pathway". Nucleic Acids Research. 38 (3): 931–941. doi:10.1093/nar/gkp1058. PMC 2817471. PMID 19933257.
- ^ a b c d e f g h i j k l m n o Fan L, Fuss JO, Cheng QJ, Arvai AS, Hammel M, Roberts VA, et al. (May 2008). "XPD helicase structures and activities: insights into the cancer and aging phenotypes from XPD mutations". Cell. 133 (5): 789–800. doi:10.1016/j.cell.2008.04.030. PMC 3055247. PMID 18510924.
- ^ a b Lainé JP, Mocquet V, Egly JM (2006). "TFIIH enzymatic activities in transcription and nucleotide excision repair". DNA Repair, Part A. Methods in Enzymology. Vol. 408. pp. 246–263. doi:10.1016/S0076-6879(06)08015-3. ISBN 9780121828134. PMID 16793373.
- ^ a b Tirode F, Busso D, Coin F, Egly JM (January 1999). "Reconstitution of the transcription factor TFIIH: assignment of functions for the three enzymatic subunits, XPB, XPD, and cdk7". Molecular Cell. 3 (1): 87–95. doi:10.1016/S1097-2765(00)80177-X. PMID 10024882.
- ^ a b Sung P, Bailly V, Weber C, Thompson LH, Prakash L, Prakash S (October 1993). "Human xeroderma pigmentosum group D gene encodes a DNA helicase". Nature. 365 (6449): 852–855. Bibcode:1993Natur.365..852S. doi:10.1038/365852a0. PMID 8413672. S2CID 4334960.
- ^ a b Schaeffer L, Roy R, Humbert S, Moncollin V, Vermeulen W, Hoeijmakers JH, et al. (April 1993). "DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor". Science. 260 (5104): 58–63. Bibcode:1993Sci...260...58S. doi:10.1126/science.8465201. PMID 8465201.
- ^ a b Hanada K, Hickson ID (September 2007). "Molecular genetics of RecQ helicase disorders". Cellular and Molecular Life Sciences. 64 (17): 2306–2322. doi:10.1007/s00018-007-7121-z. PMID 17571213. S2CID 29287970.
- ^ a b c d e Opresko PL, Cheng WH, Bohr VA (April 2004). "Junction of RecQ helicase biochemistry and human disease". The Journal of Biological Chemistry. 279 (18): 18099–18102. doi:10.1074/jbc.R300034200. PMID 15023996.
- ^ Ouyang KJ, Woo LL, Ellis NA (2008). "Homologous recombination and maintenance of genome integrity: cancer and aging through the prism of human RecQ helicases". Mechanisms of Ageing and Development. 129 (7–8): 425–440. doi:10.1016/j.mad.2008.03.003. PMID 18430459. S2CID 6804631.
- ^ Ellis NA, Groden J, Ye TZ, Straughen J, Lennon DJ, Ciocci S, et al. (November 1995). "The Bloom's syndrome gene product is homologous to RecQ helicases". Cell. 83 (4): 655–666. doi:10.1016/0092-8674(95)90105-1. PMID 7585968.
- ^ a b Selak N, Bachrati CZ, Shevelev I, Dietschy T, van Loon B, Jacob A, et al. (September 2008). "The Bloom's syndrome helicase (BLM) interacts physically and functionally with p12, the smallest subunit of human DNA polymerase delta". Nucleic Acids Research. 36 (16): 5166–5179. doi:10.1093/nar/gkn498. PMC 2532730. PMID 18682526.
- ^ a b Gray MD, Shen JC, Kamath-Loeb AS, Blank A, Sopher BL, Martin GM, et al. (September 1997). "The Werner syndrome protein is a DNA helicase". Nature Genetics. 17 (1): 100–103. doi:10.1038/ng0997-100. PMID 9288107. S2CID 20587915.
- ^ Kitao S, Shimamoto A, Goto M, Miller RW, Smithson WA, Lindor NM, Furuichi Y (May 1999). "Mutations in RECQL4 cause a subset of cases of Rothmund-Thomson syndrome". Nature Genetics. 22 (1): 82–84. doi:10.1038/8788. PMID 10319867. S2CID 195211275.
- ^ Gupta, Sonia Vidushi; Schmidt, Kristina Hildegard (2020-02-18). "Maintenance of Yeast Genome Integrity by RecQ Family DNA Helicases". Genes. 11 (2): 205. doi:10.3390/genes11020205. ISSN 2073-4425. PMC 7074392. PMID 32085395.
- ^ Debnath S., Sharma S. RECQ1 Helicase in Genomic Stability and Cancer. Genes. 2020:11. doi: 10.3390/genes11060622], Debnath S., Sharma S. RECQ1 Helicase in Genomic Stability and Cancer. Genes. 2020:11. doi: 10.3390/genes11060622].
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ Hendrickson, W. A.; Ward, K. B. (1975-10-27). "Atomic models for the polypeptide backbones of myohemerythrin and hemerythrin". Biochemical and Biophysical Research Communications. 66 (4): 1349–1356. doi:10.1016/0006-291x(75)90508-2. ISSN 1090-2104. PMID 5.
- ^ Lorenz A, Osman F, Sun W, Nandi S, Steinacher R, Whitby MC (June 2012). "The fission yeast FANCM ortholog directs non-crossover recombination during meiosis". Science. 336 (6088): 1585–1588. Bibcode:2012Sci...336.1585L. doi:10.1126/science.1220111. PMC 3399777. PMID 22723423.
- ^ Lorenz A, Mehats A, Osman F, Whitby MC (December 2014). "Rad51/Dmc1 paralogs and mediators oppose DNA helicases to limit hybrid DNA formation and promote crossovers during meiotic recombination". Nucleic Acids Research. 42 (22): 13723–13735. doi:10.1093/nar/gku1219. PMC 4267644. PMID 25414342.
- ^ a b Séguéla-Arnaud M, Crismani W, Larchevêque C, Mazel J, Froger N, Choinard S, et al. (April 2015). "Multiple mechanisms limit meiotic crossovers: TOP3α and two BLM homologs antagonize crossovers in parallel to FANCM". Proceedings of the National Academy of Sciences of the United States of America. 112 (15): 4713–4718. Bibcode:2015PNAS..112.4713S. doi:10.1073/pnas.1423107112. PMC 4403193. PMID 25825745.
- ^ a b c d Jankowsky A, Guenther UP, Jankowsky E (January 2011). "The RNA helicase database". Nucleic Acids Research. 39 (Database issue): D338–D341. doi:10.1093/nar/gkq1002. PMC 3013637. PMID 21112871.
- ^ Jankowsky E, Fairman-Williams ME (2010). "An introduction to RNA helicases: superfamilies, families, and major themes". In Jankowsky E (ed.). RNA Helicases (RSC Biomolecular Sciences). Cambridge, England: Royal Society of Chemistry. p. 5. ISBN 978-1-84755-914-2.
- ^ Ranji A, Boris-Lawrie K (2010). "RNA helicases: emerging roles in viral replication and the host innate response". RNA Biology. 7 (6): 775–787. doi:10.4161/rna.7.6.14249. PMC 3073335. PMID 21173576.
- ^ Jankowsky E (January 2011). "RNA helicases at work: binding and rearranging". Trends in Biochemical Sciences. 36 (1): 19–29. doi:10.1016/j.tibs.2010.07.008. PMC 3017212. PMID 20813532.
- ^ Yang Q, Del Campo M, Lambowitz AM, Jankowsky E (October 2007). "DEAD-box proteins unwind duplexes by local strand separation". Molecular Cell. 28 (2): 253–263. doi:10.1016/j.molcel.2007.08.016. PMID 17964264.
- ^ Liu F, Putnam A, Jankowsky E (December 2008). "ATP hydrolysis is required for DEAD-box protein recycling but not for duplex unwinding". Proceedings of the National Academy of Sciences of the United States of America. 105 (51): 20209–20214. Bibcode:2008PNAS..10520209L. doi:10.1073/pnas.0811115106. PMC 2629341. PMID 19088201.
- ^ Jarmoskaite I, Russell R (2011). "DEAD-box proteins as RNA helicases and chaperones". Wiley Interdisciplinary Reviews. RNA. 2 (1): 135–152. doi:10.1002/wrna.50. PMC 3032546. PMID 21297876.
- ^ "Index of /". www.rnahelicase.org. Archived from the original on 2014-12-18. Retrieved 2012-12-07.
- ^ Matson SW, Tabor S, Richardson CC (November 1983). "The gene 4 protein of bacteriophage T7. Characterization of helicase activity". The Journal of Biological Chemistry. 258 (22): 14017–14024. doi:10.1016/S0021-9258(17)44018-X. PMID 6315716.
- ^ Sarlós K, Gyimesi M, Kovács M (June 2012). "RecQ helicase translocates along single-stranded DNA with a moderate processivity and tight mechanochemical coupling". Proceedings of the National Academy of Sciences of the United States of America. 109 (25): 9804–9809. Bibcode:2012PNAS..109.9804S. doi:10.1073/pnas.1114468109. PMC 3382518. PMID 22665805.
- ^ Pavankumar TL, Exell JC, Kowalczykowski SC (1 January 2016). "Direct Fluorescent Imaging of Translocation and Unwinding by Individual DNA Helicases". Single-Molecule Enzymology: Fluorescence-Based and High-Throughput Methods. Methods in Enzymology. Vol. 581. pp. 1–32. doi:10.1016/bs.mie.2016.09.010. ISBN 9780128092675. PMC 5854184. PMID 27793277.
- ^ Borowiec JA (1996). "DNA Helicases". In DePamphilis ML (ed.). DNA Replication in Eukaryotic Cells. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. pp. 545–574. ISBN 978-0-87969-459-3. OCLC 246537432.
External links[edit]
- DNA+Helicases at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- RNA+Helicases at the U.S. National Library of Medicine Medical Subject Headings (MeSH)












