Regioselectivity
In chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions.[1][2] It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where on a substituted benzene ring a further substituent will add.
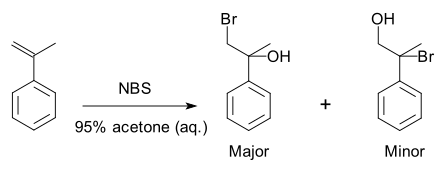
A specific example is a halohydrin formation reaction with 2-propenylbenzene[3]:
Because of the preference for the formation of one product over another, the reaction is selective. This reaction is regioselective because it selectively generates one constitutional isomer rather than the other.
Certain examples of regioselectivity have been formulated as rules for certain classes of compounds under certain conditions, many of which are named. Among the first introduced to chemistry students are Markovnikov's rule for the addition of protic acids to alkenes, and the Fürst-Plattner rule for the addition of nucleophiles to derivatives of cyclohexene, especially epoxide derivatives.[4][5]
Regioselectivity in ring-closure reactions is subject to Baldwin's rules. If there are two or more orientations that can be generated during a reaction, one of them is dominant (e.g., Markovnikov/anti-Markovnikov addition across a double bond)
Regioselectivity can also be applied to specific reactions such as addition to pi ligands.
See also
Finally, the selectivity often seen in carbene insertions or Baeyer-Villiger insertions of oxygen are regioselective, not chemoselective. Baeyer-Villiger reactions, which can be both chemical or biotransformations, exhibit selectivity because the oxygen inserts between the carbonyl and the more highly substituted carbon. In a study involving acetophenones, the oxygen preferentially inserts between the carbonyl and the aryl group to give acetic acid aryl esters, not methyl benzoates. Like the Markovnikov additions, the product depends on the stability of the intermediate compounds.
References
- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 3rd edition, New York: Wiley, ISBN 9780471854722, OCLC 642506595
- ^ http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch06/ch6-0-1.html Regioselectivity & Stereoselectivity
- ^ Regioselectivity in Organic Synthesis: Preparation of the Bromohydrin of alpha-Methylstyrene Brad Andersh, Kathryn N. Kilby, Meghan E. Turnis, and Drew L. Murphy 102 Journal of Chemical Education • Vol. 85 No. 1 January 2008
- ^ W. Markownikoff (1870). "Ueber die Abhängigkeit der verschiedenen Vertretbarkeit des Radicalwasserstoffs in den isomeren Buttersäuren". Annalen der Pharmacie 153 (1): 228–259.
- ^ Fürst, A.; Plattner, P. A. Helv. Chim. Acta 1949, 32, 275