Spider silk




Spider silk is a protein fibre spun by spiders. Spiders use their silk to make webs or other structures, which function as sticky nets to catch other animals, or as nests or cocoons to protect their offspring, or to wrap up prey. They can also use their silk to suspend themselves, to float through the air, or to glide away from predators. Most spiders vary the thickness and stickiness of their silk for different uses.
In some cases, spiders may even use silk as a source of food.[1] While methods have been developed to collect silk from a spider by force,[2] it is difficult to gather silk from many spiders compared to silk-spinning organisms such as silkworms.
All spiders produce silk, and even in non-web building spiders, silk is intimately tied to courtship and mating. Silk produced by females provides a transmission channel for male vibratory courtship signals, while webs and draglines provide a substrate for female sex pheromones. Observations of male spiders producing silk during sexual interactions are also common across phylogenetically widespread taxa. However, the function of male-produced silk in mating has received very little study.[3]
Biodiversity
Uses
All spiders produce silks, and a single spider can produce up to seven different types of silk for different uses.[4] This is in contrast to insect silks, where an individual usually only produces one type of silk.[5] Spider silks may be used in many different ecological ways, each with properties to match the silk's function. As spiders have evolved, so has their silks' complexity and diverse uses, for example from primitive tube webs 300–400 million years ago to complex orb webs 110 million years ago.[6]
| Use | Example | Reference |
|---|---|---|
| Prey capture | The orb webs produced by the Araneidae (typical orb-weavers); tube webs; tangle webs; sheet webs; lace webs, dome webs; single thread used by the Bolas spiders for "fishing". | [4][6] |
| Prey immobilisation | Silk used as "swathing bands" to wrap up prey. Often combined with immobilising prey using a venom. In species of Scytodes the silk is combined with venom and squirted from the chelicerae. | [4] |
| Reproduction | Male spiders may produce sperm webs; spider eggs are covered in silk cocoons. | [4][7] |
| Dispersal | "Ballooning" or "kiting" used by smaller spiders to float through the air, for instance for dispersal. | [8] |
| Source of food | The kleptoparasitic Argyrodes eating the silk of host spider webs. Some daily weavers of temporary webs also eat their own unused silk daily, thus mitigating a heavy metabolic expense. | [1][9] |
| Nest lining and nest construction | Tube webs used by "primitive" spiders such as the European tube web spider (Segestria florentina). Threads radiate out of nest to provide a sensory link to the outside. Silk is a component of the lids of spiders that use "trapdoors", such as members of the family Ctenizidae, and the "water" or "diving bell" spider Argyroneta aquatica builds its diving bell of silk. | [6] |
| Guide lines | Some spiders that venture from shelter will leave a trail of silk by which to find their way home again. | [9] |
| Drop lines and anchor lines | Many spiders, such as the Salticidae, that venture from shelter and leave a trail of silk, use that as an emergency line in case of falling from inverted or vertical surfaces. Many others, even web dwellers, will deliberately drop from a web when alarmed, using a silken thread as a drop line by which they can return in due course. Some, such as species of Paramystaria, also will hang from a drop line when feeding. | [9] |
| Alarm lines | Some spiders that do not spin actual trap webs do lay out alarm webs that the feet of their prey (such as ants) can disturb, cueing the spider to rush out and secure the meal if it is small enough, or to avoid contact if the intruder seems too formidable. | [9] |
| Pheromonal trails | Some wandering spiders will leave a largely continuous trail of silk impregnated with pheromones that the opposite sex can follow to find a mate. | [9] |
Types

Meeting the specification for all these ecological uses requires different types of silk suited to different broad properties, as either a fibre, a structure of fibres, or a silk-globule. These types include glues and fibres. Some types of fibres are used for structural support, others for constructing protective structures. Some can absorb energy effectively, whereas others transmit vibration efficiently. In a spider, these silk types are produced in different glands; so the silk from a particular gland can be linked to its use by the spider.
| Gland | Silk Use |
|---|---|
| Ampullate (major) | Dragline silk – used for the web's outer rim and spokes, also for the lifeline and for ballooning. |
| Ampullate (minor) | Used for temporary scaffolding during web construction. |
| Flagelliform | Capture-spiral silk – used for the capturing lines of the web. |
| Tubuliform | Egg cocoon silk – used for protective egg sacs. |
| Aciniform | Used to wrap and secure freshly captured prey; used in the male sperm webs; used in stabilimenta. |
| Aggregate | A silk glue of sticky globules. |
| Piriform | Used to form bonds between separate threads for attachment points. |
Properties
Mechanical properties
Each spider and each type of silk has a set of mechanical properties optimised for their biological function.
Most silks, in particular dragline silk, have exceptional mechanical properties. They exhibit a unique combination of high tensile strength and extensibility (ductility). This enables a silk fibre to absorb a large amount of energy before breaking (toughness, the area under a stress-strain curve).

A frequent mistake made in the mainstream media is to confuse strength and toughness, when comparing silk to other materials.[citation needed] Weight for weight, silk is stronger than steel, but not as strong as Kevlar. Spider silk is, however, tougher than both.
The variability of mechanical properties of spider silk fibres may be important and it is related to their degree of molecular alignment.[10] Mechanical properties depend strongly on the ambient conditions, i.e. humidity and temperature.[11]
Strength
A dragline silk's tensile strength is comparable to that of high-grade alloy steel (450−2000 MPa),[12][13] and about half as strong as aramid filaments, such as Twaron or Kevlar (3000 MPa).[14]
Density
Consisting of mainly protein, silks are about a sixth of the density of steel (1.3 g/cm3). As a result, a strand long enough to circle the Earth would weigh about 2 kilograms (4.4 lb). (Spider dragline silk has a tensile strength of roughly 1.3 GPa. The tensile strength listed for steel might be slightly higher – e.g. 1.65 GPa,[15][16] but spider silk is a much less dense material, so that a given weight of spider silk is five times as strong as the same weight of steel.)
Energy density
The energy density of dragline spider silk is roughly 1.2×108 J/m3.[17]
Extensibility
Silks are also extremely ductile, with some able to stretch up to five times their relaxed length without breaking.
Toughness
The combination of strength and ductility gives dragline silks a very high toughness (or work to fracture), which "equals that of commercial polyaramid (aromatic nylon) filaments, which themselves are benchmarks of modern polymer fibre technology".[18][19]
Temperature
While unlikely to be relevant in nature, dragline silks can hold their strength below -40 °C (-40 °F) and up to 220 °C (428 °F).[20] As occurs in many materials, spider silk fibres undergo a glass transition. The glass-transition temperature depends on the humidity, as water is a plasticiser for the silk.[11]
Supercontraction
When exposed to water, dragline silks undergo supercontraction, shrinking up to 50% in length and behaving like a weak rubber under tension.[11] Many hypotheses have been suggested as to its use in nature, with the most popular being to automatically tension webs built in the night using the morning dew.[citation needed]
Highest-performance
The toughest known spider silk is produced by the species Darwin's bark spider (Caerostris darwini): "The toughness of forcibly silked fibers averages 350 MJ/m3, with some samples reaching 520 MJ/m3. Thus, C. darwini silk is more than twice as tough as any previously described silk, and over 10 times tougher than Kevlar".[21]
Adhesive properties
Silk fibre is a two-compound pyriform secretion, spun into patterns (called "attachment discs") that are employed to adhere silk threads to various surfaces using a minimum of silk substrate.[22] The pyriform threads polymerise under ambient conditions, become functional immediately, and are usable indefinitely, remaining biodegradable, versatile and compatible with numerous other materials in the environment.[22] The adhesive and durability properties of the attachment disc are controlled by functions within the spinnerets.[23] Some adhesive properties of the silk resemble glue, consisting of microfibrils and lipid enclosures.[22]
Types of silk
Many species of spiders have different glands to produce silk with different properties for different purposes, including housing, web construction, defence, capturing and detaining prey, egg protection, and mobility (fine "gossamer" thread for ballooning, or for a strand allowing the spider to drop down as silk is extruded). Different specialised silks have evolved with properties suitable for different uses. For example, Argiope argentata has five different types of silk, each used for a different purpose:[24][25]
| Silk | Use |
|---|---|
| major-ampullate (dragline) silk | Used for the web's outer rim and spokes and also for the lifeline. Can be as strong per unit weight as steel, but much tougher. |
| capture-spiral (flagelliform) silk | Used for the capturing lines of the web. Sticky, extremely stretchy and tough. The capture spiral is sticky due to droplets of aggregate (a spider glue) that is placed on the spiral. The elasticity of flagelliform allows for enough time for the aggregate to adhere to the aerial prey flying into the web. |
| tubiliform (a.k.a. cylindriform) silk | Used for protective egg sacs. Stiffest silk. |
| aciniform silk | Used to wrap and secure freshly captured prey. Two to three times as tough as the other silks, including dragline. |
| minor-ampullate silk | Used for temporary scaffolding during web construction. |
Structural
Macroscopic structure down to protein hierarchy
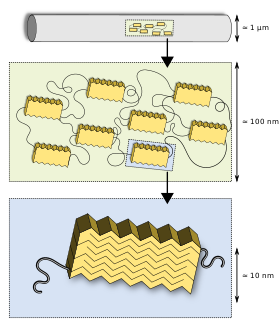
Silks, like many other biomaterials, have a hierarchical structure. The primary structure is the amino acid sequence of its proteins (spidroin), mainly consisting of highly repetitive glycine and alanine blocks,[26][27] which is why silks are often referred to as a block co-polymer. On a secondary structure level, the short side chained alanine is mainly found in the crystalline domains (beta sheets) of the nanofibril, glycine is mostly found in the so-called amorphous matrix consisting of helical and beta turn structures.[27][28] It is the interplay between the hard crystalline segments, and the strained elastic semi-amorphous regions, that gives spider silk its extraordinary properties.[29][30] Various compounds other than protein are used to enhance the fibre's properties. Pyrrolidine has hygroscopic properties which keeps the silk moist while also warding off ant invasion. It occurs in especially high concentration in glue threads. Potassium hydrogen phosphate releases hydrogen ions in aqueous solution, resulting in a pH of about 4, making the silk acidic and thus protecting it from fungi and bacteria that would otherwise digest the protein. Potassium nitrate is believed to prevent the protein from denaturing in the acidic milieu.[31]
This first very basic model of silk was introduced by Termonia in 1994[32] who suggested crystallites embedded in an amorphous matrix interlinked with hydrogen bonds. This model has refined over the years: semi-crystalline regions were found[27] as well as a fibrillar skin core model suggested for spider silk,[33] later visualised by AFM and TEM.[34] Sizes of the nanofibrillar structure and the crystalline and semi-crystalline regions were revealed by neutron scattering.[35]
It has been possible to relate microstructural information and macroscopic mechanical properties of the fibres.[36] The results show that ordered regions (i) mainly reorient by deformation for low-stretched fibres and (ii) the fraction of ordered regions increases progressively for higher stretching of the fibres.
-
Schematic of the spider's orb web, structural modules, and spider silk structure.[37] On the left is shown a schematic drawing of an orb web. The red lines represent the dragline, radial line, and frame lines, the blue lines represent the spiral line, and the centre of the orb web is called the "hub". Sticky balls drawn in blue are made at equal intervals on the spiral line with viscous material secreted from the aggregate gland. Attachment cement secreted from the piriform gland is used to connect and fix different lines. Microscopically, the spider silk secondary structure is formed of spidroin and is said to have the structure shown on the right side. In the dragline and radial line, a crystalline β-sheet and an amorphous helical structure are interwoven. The large amount of β-spiral structure gives elastic properties to the capture part of the orb web. In the structural modules diagram, a microscopic structure of dragline and radial lines is shown, composed mainly of two proteins of MaSp1 and MaSp2, as shown in the upper central part. In the spiral line, there is no crystalline β-sheet region.
Biosynthesis and fibre spinning
The production of silks, including spider silk, differs in an important aspect from the production of most other fibrous biological materials: rather than being continuously grown as keratin in hair, cellulose in the cell walls of plants, or even the fibres formed from the compacted faecal matter of beetles;[17] it is "spun" on demand from liquid silk precursor out of specialised glands.[38]
The spinning process occurs when a fibre is pulled away from the body of a spider, whether by the spider's legs, by the spider's falling under its own weight, or by any other method including being pulled by humans. The term "spinning" is misleading because no rotation of any component occurs, but rather comes from analogy to the textile spinning wheels. Silk production is a pultrusion,[39] similar to extrusion, with the subtlety that the force is induced by pulling at the finished fibre rather than being squeezed out of a reservoir. The unspun silk fibre is pulled through silk glands of which there may be both numerous duplicates and different types of gland on any one spider species.[38]
Silk gland

The gland's visible, or external, part is termed the spinneret. Depending on the complexity of the species, spiders will have two to eight spinnerets, usually in pairs. There exist highly different specialised glands in different spiders, ranging from simply a sac with an opening at one end, to the complex, multiple-section major ampullate glands of the golden silk orb-weavers.[53]
Behind each spinneret visible on the surface of the spider lies a gland, a generalised form of which is shown in the figure to the right, "Schematic of a generalised gland".

- Gland characteristics
- The first section of the gland labelled 1 on Figure 1 is the secretory or tail section of the gland. The walls of this section are lined with cells that secrete proteins Spidroin I and Spidroin II, the main components of this spider's dragline. These proteins are found in the form of droplets that gradually elongate to form long channels along the length of the final fibre, hypothesised to assist in preventing crack formation or even self-healing of the fibre.[56]
- The second section is the storage sac. This stores and maintains the gel-like unspun silk dope until it is required by the spider. In addition to storing the unspun silk gel, it secretes proteins that coat the surface of the final fibre.[18]
- The funnel rapidly reduces the large diameter of the storage sac to the small diameter of the tapering duct.
- The final length is the tapering duct, the site of most of the fibre formation. This consists of a tapering tube with several tight about turns, a valve almost at the end (mentioned in detail at point No. 5 below) ending in a spigot from which the solid silk fibre emerges. The tube here tapers hyperbolically, therefore the unspun silk is under constant elongational shear stress, which is an important factor in fibre formation. This section of the duct is lined with cells that exchange ions, reduce the dope pH from neutral to acidic, and remove water from the fibre.[57] Collectively, the shear stress and the ion and pH changes induce the liquid silk dope to undergo a phase transition and condense into a solid protein fibre with high molecular organisation. The spigot at the end has lips that clamp around the fibre, controlling fibre diameter and further retaining water.
- Almost at the end of the tapering duct is a valve, approximate position marked "5" on figure 1. Though discovered some time ago, the precise purpose of this valve is still under discussion. It is believed to assist in restarting and rejoining broken fibres,[58] acting much in the way of a helical pump, regulating the thickness of the fibre,[39] and/or clamping the fibre as a spider falls upon it.[58][59] There is some discussion of the similarity of the silk worm's silk press and the roles each of these valves play in the production of silk in these two organisms.
Throughout the process the unspun silk appears to have a nematic texture,[60] in a similar manner to a liquid crystal, arising in part due to the extremely high protein concentration of silk dope (around 30% in terms of weight per volume).[61] This allows the unspun silk to flow through the duct as a liquid but maintain a molecular order.
As an example of a complex spinning field, the spinneret apparatus of an adult Araneus diadematus (garden cross spider) consists of the glands shown below.[31] Similar multiple gland architecture exists in the black widow spider.[62]
- 500 pyriform glands for attachment points
- 4 ampullate glands for the web frame
- about 300 aciniform glands for the outer lining of egg sacs, and for ensnaring prey
- 4 tubuliform glands for egg sac silk
- 4 aggregate glands for adhesive functions
- 2 coronate glands for the thread of adhesion lines
Artificial synthesis

To artificially synthesise spider silk into fibres, there are two broad areas that must be covered. These are synthesis of the feedstock (the unspun silk dope in spiders), and synthesis of the spinning conditions (the funnel, valve, tapering duct, and spigot). There have been a number of different approaches but few of these methods have produced silk that can efficiently be synthesised into fibres.
Feedstock
The molecular structure of unspun silk is both complex and extremely long. Though this endows the silk fibres with their desirable properties, it also makes replication of the fibre somewhat of a challenge. Various organisms have been used as a basis for attempts to replicate some components or all of some or all of the proteins involved. These proteins must then be extracted, purified and then spun before their properties can be tested.
| Organism | Details | Average Maximum breaking stress (MPa) | Average Strain (%) | Reference |
|---|---|---|---|---|
| Darwin's bark spider (Caerostris darwini) | Malagasy spider famed for making webs with strands up to 25 m long, across rivers. "C. darwini silk is more than twice as tough as any previously described silk" | 1850 ±350 | 33 ±0.08 | [21] |
| Nephila clavipes | Typical golden orb weaving spider | 710–1200 | 18–27 | [63][64] |
| Bombyx mori Silkworms | Silkworms were genetically altered to express spider proteins and fibres measured.[65] | 660 | 18.5 | [66] |
| E. coli | Synthesising a large and repetitive molecule (~300 kDa) is complex, but required for the strongest silk. Here E. coli was engineered to produce a 556 kDa protein. Fibers spun from these synthetic spidroins are the first to fully replicate the mechanical performance of natural spider silk by all common metrics. | 1030 ±110 | 18 ±6 | [67] |
| Goats | Goats were genetically modified to secrete silk proteins in their milk, which could then be purified. | 285–250 | 30–40 | [68] |
| Tobacco & potato plants | Tobacco and potato plants were genetically modified to produce silk proteins. Patents were granted,[69] but no fibres have yet been described in the literature. | n/a | n/a | [70] |
Geometry
Spider silks with comparatively simple molecular structure need complex ducts to be able to spin an effective fibre. There have been a number of methods used to produce fibres, of which the main types are briefly discussed below.
Syringe and needle
Feedstock is simply forced through a hollow needle using a syringe. This method has been shown to make fibres successfully on multiple occasions.[71][72]
Although very cheap and easy to produce, the shape and conditions of the gland are very loosely approximated. Fibres created using this method may need encouragement to change from liquid to solid by removing the water from the fibre with such chemicals as the environmentally undesirable methanol[73] or acetone,[72] and also may require post-stretching of the fibre to attain fibres with desirable properties.[74][71]
Microfluidics
As the field of microfluidics matures, it is likely that more attempts to spin fibres will be made using microfluidics. These have the advantage of being very controllable and able to test spin very small volumes of unspun fibre[75][76] but setup and development costs are likely to be high. A patent has been granted in this area for spinning fibres in a method mimicking the process found in nature, and fibres are successfully being continuously spun by a commercial company.[77]
Electrospinning
Electrospinning is a very old technique whereby a fluid is held in a container in a manner such that it is able to flow out through capillary action. A conducting substrate is positioned below, and a large difference in electrical potential is applied between the fluid and the substrate. The fluid is attracted to the substrate, and tiny fibres jump almost instantly from their point of emission, the Taylor cone, to the substrate, drying as they travel. This method has been shown to create nano-scale fibres from both silk dissected from organisms and regenerated silk fibroin.
Other artificial shapes formed from silk
Silk can be formed into other shapes and sizes such as spherical capsules for drug delivery, cell scaffolds and wound healing, textiles, cosmetics, coatings, and many others.[78][79] Spider silk proteins can also self-assemble on superhydrophobic surfaces to generate nanowires, as well as micron-sized circular sheets.[79] It has recently been shown that recombinant spider silk proteins can self-assemble at the liquid air interface of a standing solution to form protein permeable, strong, and flexible nanomembranes that support cell proliferation. Suggested applications include skin transplants, and supportive membranes in organ-on-a-chip.[80] These spider silk nanomembranes have also been used to create a static in-vitro model of a blood vessel.[81]
Research milestones
Due to spider silk being a scientific research field with a long and rich history, there can be unfortunate occurrences of researchers independently rediscovering previously published findings. What follows is a table of the discoveries made in each of the constituent areas, acknowledged by the scientific community as being relevant and significant by using the metric of scientific acceptance, citations. Thus, only papers with 50 or more citations are included.
| Area of contribution | Year | Main researchers [Ref] | Title of paper | Contribution to the field |
|---|---|---|---|---|
| Chemical Basis | 1960 | Fischer, F. & Brander, J.[82] | "Eine Analyse der Gespinste der Kreuzspinne" (Amino acid composition analysis of spider silk) | |
| 1960 | Lucas, F. et al.[83][84] | "The Composition of Arthropod Silk Fibroins; Comparative studies of fibroins" | ||
| Gene Sequence | 1990 | Xu, M. & Lewis, R. V.[85] | "Structure of a Protein Superfiber − Spider Dragline Silk" | |
| Mechanical Properties | 1964 | Lucas, F.[86] | "Spiders and their silks" | First time compared mechanical properties of spider silk with other materials in a scientific paper. |
| 1989 | Vollrath, F. & Edmonds, D. T.[87] | "Modulation of the Mechanical Properties of Spider Silk by Coating with Water" | First important paper suggesting the water interplay with spider silk fibroin modulating the properties of silk. | |
| 2001 | Vollrath, F. & Shao, Z.Z.[88] | "The effect of spinning conditions on the mechanics of a spider's dragline silk" | ||
| 2006 | Plaza, G.R., Guinea, G.V., Pérez-Rigueiro, J. & Elices, M.[11] | "Thermo-hygro-mechanical behavior of spider dragline silk: Glassy and rubbery states" | Combined effect of humidity and temperature on the mechanical properties. Glass-transition temperature dependence on humidity. | |
| Structural Characterisation | 1992 | Hinman, M.B. & Lewis, R. V[26] | "Isolation of a clone encoding a second dragline silk fibroin. Nephila clavipes dragline silk is a two-protein fiber" | |
| 1994 | Simmons, A. et al.[89] | "Solid-State C-13 Nmr of Nephila-Clavipes Dragline Silk Establishes Structure and Identity of Crystalline Regions" | First NMR study of spider silk. | |
| 1999 | Shao, Z., Vollrath, F. et al.[90] | "Analysis of spider silk in native and supercontracted states using Raman spectroscopy" | First Raman study of spider silk. | |
| 1999 | Riekel, C., Muller, M.et al.[91] | "Aspects of X-ray diffraction on single spider fibers" | First X-ray on single spider silk fibres. | |
| 2000 | Knight, D.P., Vollrath, F. et al.[92] | "Beta transition and stress-induced phase separation in the spinning of spider dragline silk" | Secondary structural transition confirmation during spinning. | |
| 2001 | Riekel, C. & Vollrath, F.[93] | "Spider silk fibre extrusion: combined wide- and small-angle X- ray microdiffraction experiments" | First X-ray on spider silk dope. | |
| 2002 | Van Beek, J. D. et al.[28] | "The molecular structure of spider dragline silk: Folding and orientation of the protein backbone" | ||
| Structure-Property Relationship | 1986 | Gosline, G.M. et al.[94] | "The structure and properties of spider silk" | First attempt to link structure with properties of spider silk |
| 1994 | Termonia, Y[32] | "Molecular Modeling of Spider Silk Elasticity" | X-ray evidence presented in this paper; simple model of crystallites embedded in amorphous regions. | |
| 1996 | Simmons, A. et al.[27] | "Molecular orientation and two-component nature of the crystalline fraction of spider dragline silk" | Two types of alanine-rich crystalline regions were defined. | |
| 2006 | Vollrath, F. & Porter, D.[95] | "Spider silk as an archetypal protein elastomer" | New insight and model to spider silk based on Group Interaction Modelling. | |
| Native Spinning | 1991 | Kerkam, K., Kaplan, D. et al.[96] | "Liquid Crystallinity of Natural Silk Secretions" | |
| 1999 | Knight, D.P. & Vollrath, F.[97] | "Liquid crystals and flow elongation in a spider's silk production line" | ||
| 2001 | Vollrath, F. & Knight, D.P.[17] | "Liquid crystalline spinning of spider silk" | The most cited paper on spider silk | |
| 2005 | Guinea, G.V., Elices, M., Pérez-Rigueiro, J. & Plaza, G.R.[10] | "Stretching of supercontracted fibers: a link between spinning and the variability of spider silk" | Explanation of the variability of mechanical properties. | |
| Reconstituted /Synthetic Spider Silk and Artificial Spinning | 1995 | Prince, J. T., Kaplan, D. L. et al.[98] | "Construction, Cloning, and Expression of Synthetic Genes Encoding Spider Dragline Silk" | First successful synthesis of Spider silk by E. coli. |
| 1998 | Arcidiacono, S., Kaplan, D.L. et al.[99] | "Purification and characterization of recombinant spider silk expressed in Escherichia coli" | ||
| 1998 | Seidel, A., Jelinski, L.W. et al.[100] | "Artificial Spinning of Spider Silk" | First controlled wet-spinning of reconstituted spider silk. |
Human uses

Peasants in the southern Carpathian Mountains used to cut up tubes built by Atypus and cover wounds with the inner lining. It reportedly facilitated healing, and even connected with the skin. This is believed to be due to antiseptic properties of spider silk[102] and because the silk is rich in vitamin K, which can be effective in clotting blood.[103][verify] Due to the difficulties in extracting and processing substantial amounts of spider silk, the largest known piece of cloth made of spider silk is an 11-by-4-foot (3.4 by 1.2 m) textile with a golden tint made in Madagascar in 2009.[104] Eighty-two people worked for four years to collect over one million golden orb spiders and extract silk from them.[105]
The silk of Nephila clavipes was used in research concerning mammalian neuronal regeneration.[106]
Spider silk has been used as a thread for crosshairs in optical instruments such as telescopes, microscopes,[107] and telescopic rifle sights.[108] In 2011, spider silk fibres were used in the field of optics to generate very fine diffraction patterns over N-slit interferometric signals used in optical communications.[109] In 2012, spider silk fibres were used to create a set of violin strings.[110]
Development of methods to mass-produce spider silk has led to manufacturing of military, medical and consumer goods, such as ballistics armour, athletic footwear, personal care products, breast implant and catheter coatings, mechanical insulin pumps, fashion clothing, and outerwear.[111]
Spider silk is used to suspend inertial confinement fusion targets during laser ignition, as it remains considerably elastic and has a high energy to break at temperatures as low as 10–20 K. In addition, it is made from "light" atomic number elements that won't emit x-rays during irradiation that could preheat the target so that the pressure differential required for fusion is not achieved.[112]
Spider silk has been used to create biolenses that could be used in conjunction with lasers to create high-resolution images of the inside of the human body.[113]
Attempts at producing synthetic spider silk
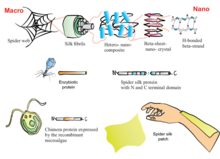
Replicating the complex conditions required to produce fibres that are comparable to spider silk has proven difficult in research and early-stage manufacturing. Through genetic engineering, Escherichia coli bacteria, yeasts, plants, silkworms, and animals other than silkworms have been used to produce spider silk proteins, which have different, simpler characteristics than those from a spider.[111] Extrusion of protein fibres in an aqueous environment is known as "wet-spinning". This process has so far produced silk fibres of diameters ranging from 10 to 60 μm, compared to diameters of 2.5–4 μm for natural spider silk. Artificial spider silks have fewer and simpler proteins than natural dragline silk, and are consequently half the diameter, strength, and flexibility of natural dragline silk.[111]
- In March 2010, researchers from the Korea Advanced Institute of Science & Technology succeeded in making spider silk directly using the bacteria E. coli, modified with certain genes of the spider Nephila clavipes. This approach eliminates the need to milk spiders and allows the manufacture of the spider silk in a more cost-effective manner.[114]
- A 556 kDa spider silk protein was manufactured from 192 repeat motifs of the Nephila clavipes dragline spidroin, having similar mechanical characteristics as their natural counterparts, i.e., tensile strength (1.03 ± 0.11 GPa), modulus (13.7 ± 3.0 GPa), extensibility (18 ± 6%), and toughness (114 ± 51 MJ/m3).[67]
- The company AMSilk developed spidroin using bacteria, making it into an artificial spider silk.[111][115]
- The company Bolt Threads produces a recombinant spidroin using yeast, for use in apparel fibers and personal care. They produced the first commercial apparel products made of recombinant spider silk, trademarked Microsilk, demonstrated in ties and beanies. They have also partnered with vegan activist and luxury designer Stella McCartney as well as Adidas to produce Microsilk garments.[116][117]
- The company Kraig Biocraft Laboratories used research from the Universities of Wyoming and Notre Dame to create silkworms that were genetically altered to produce spider silk.[118][119]
- The now defunct Canadian biotechnology company Nexia successfully produced spider silk protein in transgenic goats that carried the gene for it; the milk produced by the goats contained significant quantities of the protein, 1–2 grams of silk proteins per litre of milk. Attempts to spin the protein into a fibre similar to natural spider silk resulted in fibres with tenacities of 2–3 grams per denier.[120] Nexia used wet spinning and squeezed the silk protein solution through small extrusion holes in order to simulate the behavior of the spinneret, but this procedure was not sufficient to replicate the stronger properties of native spider silk.[121]
- The company Spiber has produced a synthetic spider silk that they are calling Q/QMONOS. In partnership with Goldwin, a ski parka made from this synthetic spider silk is currently in testing and is to be in mass production soon for less than $120,000 YEN.[122][123]
References
- ^ a b Miyashita, Tadashi; Maezono, Yasunori; Shimazaki, Aya (2004). "Silk feeding as an alternative foraging tactic in a kleptoparasitic spider under seasonally changing environments" (PDF). Journal of Zoology. 262 (3): 225–29. CiteSeerX 10.1.1.536.9091. doi:10.1017/S0952836903004540.
- ^ Work, Robert W.; Emerson, Paul D. (1982). "An Apparatus and Technique for the Forcible Silking of Spiders". Journal of Arachnology. 10 (1): 1–10. JSTOR 3705113.
- ^ Scott, Catherine E.; Anderson, Alissa G.; Andrade, Maydianne C. B. (August 2018). "A review of the mechanisms and functional roles of male silk use in spider courtship and mating". The Journal of Arachnology. 46 (2): 173–206. doi:10.1636/JoA-S-17-093.1. ISSN 0161-8202. S2CID 53322197.
- ^ a b c d Foelix, R. F. (1996). Biology of Spiders. Oxford; New York: Oxford University Press. p. 330. ISBN 978-0-19-509594-4.
- ^ Sutherland, TD; Young, JH; Weisman, S; Hayashi, CY; Merritt, DJ (2010). "Insect silk: One name, many materials". Annual Review of Entomology. 55: 171–88. doi:10.1146/annurev-ento-112408-085401. PMID 19728833.
- ^ a b c Hillyard, P. (2007). The Private Life of Spiders. London: New Holland. p. 160. ISBN 978-1-84537-690-1.
- ^ Nentwig, Wolfgang; Heimer, Stefan (1987). "Ecological Aspects of Spider Webs". Ecophysiology of Spiders. pp. 211–225. doi:10.1007/978-3-642-71552-5_15. ISBN 978-3-642-71554-9.
- ^ Flying spiders over Texas! Coast to Coast. Chad B., Texas State University Undergrad Archived 26 November 2011 at the Wayback Machine Describes the mechanical kiting of Spider "ballooning".
- ^ a b c d e Holm, Erik, Dippenaar-Schoeman, Ansie; Goggo Guide; LAPA publishers (URL: WWW.LAPA.co.za). 2010[page needed]
- ^ a b Guinea, G.V.; Elices, M.; Pérez-Rigueiro, J. & Plaza, G.R. (2005). "Stretching of supercontracted fibers: a link between spinning and the variability of spider silk". Journal of Experimental Biology. 208 (1): 25–30. doi:10.1242/jeb.01344. PMID 15601874.
- ^ a b c d Plaza, Gustavo R.; Guinea, Gustavo V.; Pérez-Rigueiro, José; Elices, Manuel (2006). "Thermo-hygro-mechanical behavior of spider dragline silk: Glassy and rubbery states". Journal of Polymer Science Part B: Polymer Physics. 44 (6): 994–99. Bibcode:2006JPoSB..44..994P. doi:10.1002/polb.20751.
- ^ Griffiths, J. R.; Salanitri, V. R. (1980). "The strength of spider silk". Journal of Materials Science. 15 (2): 491–96. Bibcode:1980JMatS..15..491G. doi:10.1007/BF00551703. S2CID 135628690.
- ^ "Overview of materials for AISI 4000 Series Steel". matweb.com. Retrieved 18 August 2010.
- ^ "DuPont Kevlar 49 Aramid Fiber". matweb.com. Retrieved 18 August 2010.
- ^ Ganio Mego, Paolo (c. 2002). "Material Tensile Strength Comparison". Archived from the original on 26 October 2009. Retrieved 3 January 2012.
- ^ Shao, Zhengzhong; Vollrath, F (2002). "Materials: Surprising strength of silkworm silk". Nature. 418 (6899): 741. Bibcode:2002Natur.418..741S. doi:10.1038/418741a. PMID 12181556. S2CID 4304912.
- ^ a b c Porter, D.; Vollrath, F.; Shao, Z. (2005). "Predicting the mechanical properties of spider silk as a model nanostructured polymer". European Physical Journal E. 16 (2): 199–206. Bibcode:2005EPJE...16..199P. doi:10.1140/epje/e2005-00021-2. PMID 15729511. S2CID 32385814.
- ^ a b Vollrath, F. & Knight, D. P. (2001). "Liquid crystalline spinning of spider silk". Nature. 410 (6828): 541–48. Bibcode:2001Natur.410..541V. doi:10.1038/35069000. PMID 11279484. S2CID 205015549.
- ^ "Spider Silk". chm.bris.ac.uk. Retrieved 18 August 2010.
- ^ Yang, Y.; Chen, X.; Shao, Z.; Zhou, P.; Porter, D.; Knight, D. P.; Vollrath, F. (2005). "Toughness of Spider Silk at High and Low Temperatures". Advanced Materials. 17: 84–88. doi:10.1002/adma.200400344. S2CID 136693986.
- ^ a b Agnarsson, Ingi; Kuntner, Matjaž; Blackledge, Todd A. (2010). Lalueza-Fox, Carles (ed.). "Bioprospecting Finds the Toughest Biological Material: Extraordinary Silk from a Giant Riverine Orb Spider". PLOS ONE. 5 (9): 11234. Bibcode:2010PLoSO...511234A. doi:10.1371/journal.pone.0011234. PMC 2939878. PMID 20856804.

- ^ a b c Wolff, J. O.; Grawe, I; Wirth, M; Karstedt, A; Gorb, S. N. (2015). "Spider's super-glue: Thread anchors are composite adhesives with synergistic hierarchical organization". Soft Matter. 11 (12): 2394–403. Bibcode:2015SMat...11.2394W. doi:10.1039/c4sm02130d. PMID 25672841.
- ^ Sahni, V; Harris, J; Blackledge, T. A.; Dhinojwala, A (2012). "Cobweb-weaving spiders produce different attachment discs for locomotion and prey capture". Nature Communications. 3: 1106. Bibcode:2012NatCo...3.1106S. doi:10.1038/ncomms2099. PMID 23033082.
- ^ Cunningham, Aimee (2009). "Taken for a spin: Scientists look to spiders for the goods on silk". Science News. 171 (15): 231–34. doi:10.1002/scin.2007.5591711509.
- ^ Blackledge, TA; Hayashi, CY (2006). "Silken toolkits: Biomechanics of silk fibers spun by the orb web spider Argiope argentata (Fabricius 1775)". The Journal of Experimental Biology. 209 (Pt 13): 2452–61. doi:10.1242/jeb.02275. PMID 16788028.
- ^ a b Hinman, M. B. & Lewis, R. V. (1992). "Isolation of a clone encoding a second dragline silk fibroin. Nephila clavipes dragline silk is a two-protein fiber". J. Biol. Chem. 267 (27): 19320–24. doi:10.1016/S0021-9258(18)41777-2. PMID 1527052.
- ^ a b c d Simmons, A. H.; Michal, C. A. & Jelinski, L. W. (1996). "Molecular orientation and two-component nature of the crystalline fraction of spider dragline silk". Science. 271 (5245): 84–87. Bibcode:1996Sci...271...84S. doi:10.1126/science.271.5245.84. PMID 8539605. S2CID 40043335.
- ^ a b van Beek, J. D.; Hess, S.; Vollrath, F. & Meier, B. H. (2002). "The molecular structure of spider dragline silk: Folding and orientation of the protein backbone". Proc. Natl. Acad. Sci. U.S.A. 99 (16): 10266–71. Bibcode:2002PNAS...9910266V. doi:10.1073/pnas.152162299. PMC 124902. PMID 12149440.
- ^ Liu, Y.; Sponner, A.; Porter, D.; Vollrath, F. (2008). "Proline and Processing of Spider Silks". Biomacromolecules. 9 (1): 116–21. doi:10.1021/bm700877g. PMID 18052126.
- ^ Papadopoulos, P.; Ene, R.; Weidner, I.; Kremer, F. (2009). "Similarities in the Structural Organization of Major and Minor Ampullate Spider Silk". Macromol. Rapid Commun. 30 (9–10): 851–57. doi:10.1002/marc.200900018. PMID 21706668.
- ^ a b Heimer, S. (1988). Wunderbare Welt der Spinnen. Urania. p. 12
- ^ a b Termonia, Y. (1994). "Molecular Modeling of Spider Silk Elasticity". Macromolecules. 27 (25): 7378–81. Bibcode:1994MaMol..27.7378T. doi:10.1021/ma00103a018.
- ^ Vollrath, F.; Holtet, T.; Thogersen, H. C. & Frische, S. (1996). "Structural organization of spider silk". Proceedings of the Royal Society B. 263 (1367): 147–51. Bibcode:1996RSPSB.263..147V. doi:10.1098/rspb.1996.0023. S2CID 136879037.
- ^ Sponner, A.; Vater, Wolfram, Wolfram; Monajembashi, Shamci, Shamci; Unger, Eberhard, Eberhard; Grosse, Frank, Frank; Weisshart, Klaus, Klaus (2007). Scheibel, Thomas (ed.). "Composition and hierarchical organization of a spider silk". PLOS ONE. 2 (10): e998. Bibcode:2007PLoSO...2..998S. doi:10.1371/journal.pone.0000998. PMC 1994588. PMID 17912375.

- ^ Sapede, D.; Seydel, T.; Forsyth, V. T.; Koza, M. M.; Schweins, R.; Vollrath, F.; Riekel, C. (2005). "Nanofibrillar structure and molecular mobility in spider dragline silk". Macromolecules. 34 (20): 623. Bibcode:2005MaMol..38.8447S. doi:10.1021/ma0507995.
- ^ Plaza, G.R.; Pérez-Rigueiro, J.; Riekel, C.; Perea, G.B.; Agulló-Rueda, F.; Burghammer, M.; Guinea, G.V.; Elices, M. (2012). "Relationship between microstructure and mechanical properties in spider silk fibers: identification of two regimes in the microstructural changes". Soft Matter. 8 (22): 6015–26. Bibcode:2012SMat....8.6015P. doi:10.1039/C2SM25446H.
- ^ Zhao, Yue; Hien, Khuat Thi Thu; Mizutani, Goro; Rutt, Harvey N. (June 2017). "Second-order nonlinear optical microscopy of spider silk". Applied Physics B. 123 (6): 188. arXiv:1706.03186. Bibcode:2017ApPhB.123..188Z. doi:10.1007/s00340-017-6766-z. S2CID 51684427.
- ^ a b Andersson, M; Johansson, J; Rising, A (2016). "Silk Spinning in Silkworms and Spiders". International Journal of Molecular Sciences. 17 (8): 1290. doi:10.3390/ijms17081290. PMC 5000687. PMID 27517908.
- ^ a b Wilson, R. S. (1969). "control of drag-line spinning in certain spiders". Am. Zool. 9: 103–. doi:10.1093/icb/9.1.103.
- ^ Zhao, Yue; Li, Yanrong; Hien, K. T. T.; Mizutani, Goro; Rutt, Harvey N. (2019). "Observation of Spider Silk by Femtosecond Pulse Laser Second Harmonic Generation Microscopy". Surf. Interface Anal. 51 (1): 50–56. arXiv:1812.10390. doi:10.1002/sia.6545. S2CID 104921418.
- ^ a b Rising, A.; Johansson, J. (2015). "Toward spinning artificial spider silk". Nat. Chem. Biol. 11 (5): 309–15. doi:10.1038/nchembio.1789. PMID 25885958.
- ^ a b c Eisoldt, L.; Thamm, C.; Scheibel, T. (2012). "The role of terminal domains during storage and assembly of spider silk proteins". Biopolymers. 97 (6): 355–61. doi:10.1002/bip.22006. PMID 22057429. S2CID 46685716.
- ^ Eisoldt, L.; Smith, A.; Scheibel, T. (2011). "Decoding the secrets of spider silk". Mater. Today. 14 (3): 80–86. doi:10.1016/S1369-7021(11)70057-8.
- ^ Tokareva, O.; Jacobsen, M.; Buehler, M.; Wong, J.; Kaplan, D. L. (2014). "Structure–function–property–design interplay in biopolymers: Spider silk". Acta Biomater. 10 (4): 1612–26. doi:10.1016/j.actbio.2013.08.020. PMC 3926901. PMID 23962644.
- ^ a b Vollrath, F.; Knight, D. P. (2001). "Liquid crystalline spinning of spider silk". Nature. 410 (6828): 541–48. Bibcode:2001Natur.410..541V. doi:10.1038/35069000. PMID 11279484. S2CID 205015549.
- ^ a b Kluge, J. A.; Rabotyagova, O.; Leisk, G. G.; Kaplan, D. L. (2008). "Spider silks and their applications". Trends Biotechnol. 26 (5): 244–51. doi:10.1016/j.tibtech.2008.02.006. PMID 18367277.
- ^ Hijirida, D. H.; Do, K. G.; Michal, C.; Wong, S.; Zax, D.; Jelinski, L. W. (1996). "13C NMR of Nephila clavipes major ampullate silk gland". Biophys. J. 71 (6): 3442–47. Bibcode:1996BpJ....71.3442H. doi:10.1016/S0006-3495(96)79539-5. PMC 1233831. PMID 8968613.
- ^ Lefvre, T.; Boudreault, S.; Cloutier, C.; Pezolet, M. (2008). "Conformational and orientational transformation of silk proteins in the major ampullate gland of Nephila clavipes spiders". Biomacromolecules. 9 (9): 2399–407. doi:10.1021/bm800390j. PMID 18702545.
- ^ Lewis, R. V. (2006). "Spider silk: Ancient ideas for new biomaterials". Chem. Rev. 106 (9): 3762–74. doi:10.1021/cr010194g. PMID 16967919.
- ^ Andersson, M.; et al. (2014). "Carbonic anhydrase generates CO2 and H+ that drive spider silk formation via opposite effects on the terminal domains". PLOS Biol. 12 (8): e1001921. doi:10.1371/journal.pbio.1001921. PMC 4122339. PMID 25093327.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Kronqvist, N.; et al. (2014). "Sequential pH-driven dimerization and stabilization of the N-terminal domain enables rapid spider silk formation". Nat. Commun. 5: 3254. Bibcode:2014NatCo...5.3254K. doi:10.1038/ncomms4254. PMID 24510122.
- ^ Knight, D. P.; Vollrath, F. (1999). "Liquid crystals and flow elongation in a spider's silk production line". Proc. R. Soc. B. 266 (1418): 519–23. doi:10.1098/rspb.1999.0667. PMC 1689793.
- ^ Dicko, C.; Porter, D.; Bond, J.; Kenney, J. M. & Vollratht, F. (2008). "Structural disorder in silk proteins reveals the emergence of elastomericity". Biomacromolecules. 9 (1): 216–21. doi:10.1021/bm701069y. PMID 18078324.
- ^ Lefèvre, T.; Boudreault, S.; Cloutier, C. & Pézolet, M. (2008). "Conformational and orientational transformation of silk proteins in the major ampullate gland of Nephila clavipes spiders". Biomacromolecules. 9 (9): 2399–407. doi:10.1021/bm800390j. PMID 18702545.
- ^ Heim, M.; Keerl, D. & Scheibel, T. (2009). "Spider Silk: From Soluble Protein to Extraordinary Fiber". Angewandte Chemie International Edition. 48 (20): 3584–96. doi:10.1002/anie.200803341. PMID 19212993.
- ^ Heinhorst, S.; Cannon, G. (2002). "Nature: Self-Healing Polymers and Other Improved Materials". J. Chem. Educ. 79 (1): 10. Bibcode:2002JChEd..79...10H. doi:10.1021/ed079p10.
- ^ Knight, D. P.; Vollrath, F. (1 April 2001). "Changes in element composition along the spinning duct in a Nephila spider". Die Naturwissenschaften. 88 (4): 179–82. Bibcode:2001NW.....88..179K. doi:10.1007/s001140100220. ISSN 0028-1042. PMID 11480706. S2CID 26097179.
- ^ a b Vollrath, F. & Knight, D. P. (1998). "Structure and function of the silk production pathway in spider Nephila edulis". Int J Biol Macromol. 24 (2–3): 243–49. doi:10.1016/S0141-8130(98)00095-6. PMID 10342771.
- ^ Wilson, R. S. (1962). "The Control of Dragline Spinning in the Garden Spider". Quarterly Journal of Microscopical Science. 103: 557–71.
- ^ Magoshi, J.; Magoshi, Y. & Nakamura, S. (1985). "Physical properties and structure of silk: 9. Liquid crystal formation of silk fibroin". Polym. Commun. 26: 60–61.
- ^ Chen, Xin; Knight, David P.; Vollrath, Fritz (1 July 2002). "Rheological characterization of nephila spidroin solution". Biomacromolecules. 3 (4): 644–48. doi:10.1021/bm0156126. ISSN 1525-7797. PMID 12099805.
- ^ Jeffery, F; La Mattina, C; Tuton-Blasingame, T; Hsia, Y; Gnesa, E; Zhao, L; Franz, A; Vierra, C (2011). "Microdissection of Black Widow Spider Silk-producing Glands". Journal of Visualized Experiments (47): 2382. doi:10.3791/2382. PMC 3341101. PMID 21248709.
- ^ Elices, M.; Plaza, G.R.; Arnedo, M.A.; Perez-Rigueiro, J.; Torres, F.G. & Guinea, G. (2009). "Mechanical Behavior of Silk During the Evolution of Orb-Web Spinning Spiders". Biomacromolecules. 10 (7): 1904–10. doi:10.1021/bm900312c. PMID 19505138.
- ^ Swanson, B. O.; Blackledge, T. A.; Summers, A. P. & Hayashi, C. Y. (2006). "Spider dragline silk: Correlated and mosaic evolution in high-performance biological materials" (PDF). Evolution. 60 (12): 2539–51. doi:10.1554/06-267.1. PMID 17263115. S2CID 14862626.
- ^ Shao, Z. Z. & Vollrath, F. (2002). "Materials: Surprising strength of silkworm silk". Nature. 418 (6899): 741. Bibcode:2002Natur.418..741S. doi:10.1038/418741a. PMID 12181556. S2CID 4304912.
- ^ Wen, H. X.; et al. (2010). "Transgenic silkworms (Bombyx mori) produce recombinant spider dragline silk in cocoons". Molecular Biology Reports. 37 (4): 1815–21. doi:10.1007/s11033-009-9615-2. PMID 19633923. S2CID 12924107.
- ^ a b Bowen, C.H. (2018). "Recombinant Spidroins Fully Replicate Primary Mechanical Properties of Natural Spider Silk". Biomacromolecules. 19 (9): 3853–60. doi:10.1021/acs.biomac.8b00980. hdl:2060/20180007385. PMID 30080972. S2CID 51930371.
- ^ Elices, M.; Guinea, G. V.; Plaza, G. R.; Karatzas, C.; Riekel, C.; Agulló-Rueda, F.; Daza, R.; Pérez-Rigueiro, J. (2011). "Bioinspired Fibers Follow the Track of Natural Spider Silk". Macromolecules. 44 (5): 1166–76. Bibcode:2011MaMol..44.1166E. doi:10.1021/ma102291m. S2CID 97699665.
- ^ US patent 2008109923, Lewis, R. V., "Expression of spider silk proteins", published 2010-05-25, assigned to University of Wyoming
- ^ Scheller, J. & Conrad, U. (2005). "Plant-based material, protein and biodegradable plastic". Current Opinion in Plant Biology. 8 (2): 188–96. doi:10.1016/j.pbi.2005.01.010. PMID 15753000.
- ^ a b Lazaris, A.; Arcidiacono, S, S; Huang, Y, Y; Zhou, J. F., JF; Duguay, F, F; Chretien, N, N; Welsh, E. A., EA; Soares, J. W., JW; Karatzas, C. N., CN (2002). "Spider silk fibers spun from soluble recombinant silk produced in mammalian cells". Science. 295 (5554): 472–76. Bibcode:2002Sci...295..472L. doi:10.1126/science.1065780. PMID 11799236. S2CID 9260156.
- ^ a b Seidel, A.; Liivak, Oskar; Calve, Sarah; Adaska, Jason; Ji, Gending; Yang, Zhitong; Grubb, David; Zax, David B.; Jelinski, Lynn W. (2000). "Regenerated spider silk: Processing, properties, and structure". Macromolecules. 33 (3): 775–80. Bibcode:2000MaMol..33..775S. doi:10.1021/ma990893j.
- ^ Arcidiacono, S.; Mello, Charlene M.; Butler, Michelle; Welsh, Elizabeth; Soares, Jason W.; Allen, Alfred; Ziegler, David; Laue, Thomas; Chase, Susan (2002). "Aqueous processing and fiber spinning of recombinant spider silks". Macromolecules. 35 (4): 1262–66. Bibcode:2002MaMol..35.1262A. doi:10.1021/ma011471o.
- ^ Xia, X. X.; et al. (2010). "Native-sized recombinant spider silk protein produced in metabolically engineered Escherichia coli results in a strong fiber". Proceedings of the National Academy of Sciences of the United States of America. 107 (32): 14, 059–63. Bibcode:2010PNAS..10714059X. doi:10.1073/pnas.1003366107. PMC 2922564. PMID 20660779.
- ^ Kinahan, M. E.; et al. (2011). "Tunable Silk: Using Microfluidics to Fabricate Silk Fibers with Controllable Properties". Biomacromolecules. 12 (5): 1504–11. doi:10.1021/bm1014624. PMC 3305786. PMID 21438624.
- ^ Rammensee, S.; Slotta, U.; Scheibel, T. & Bausch, A. R. (2008). "Assembly mechanism of recombinant spider silk proteins (microfluidic)". Proceedings of the National Academy of Sciences of the United States of America. 105 (18): 6590–95. Bibcode:2008PNAS..105.6590R. doi:10.1073/pnas.0709246105. PMC 2373321. PMID 18445655.
- ^ Spintec Engineering GmbH (in German)
- ^ Eisoldt, L.; Smith, A. & Scheibel, T. (2011). "Decoding the secrets of spider silk". Mater. Today. 14 (3): 80–86. doi:10.1016/s1369-7021(11)70057-8.
- ^ a b Gustafsson, L.; Jansson, R.; Hedhammar, M. & van der Wijngaart, W. (2018). "Structuring of Functional Spider Silk Wires, Coatings, and Sheets by Self-Assembly on Superhydrophobic Pillar Surfaces". Adv. Mater. 30 (3): 1704325. doi:10.1002/adma.201704325. PMID 29205540. S2CID 205283504.
- ^ Gustafsson, Linnea; Panagiotis Tasiopoulos, Christos; Jansson, Ronnie; Kvick, Mathias; Duursma, Thijs; Gasser, Thomas Christian; van der Wijngaart, Wouter; Hedhammar, My (16 August 2020). "Recombinant Spider Silk Forms Tough and Elastic Nanomembranes that are Protein‐Permeable and Support Cell Attachment and Growth". Advanced Functional Materials. 30 (40): 2002982. doi:10.1002/adfm.202002982.
- ^ Tasiopoulos, Christos Panagiotis; Gustafsson, Linnea; van der Wijngaart, Wouter; Hedhammar, My (25 June 2021). "Fibrillar Nanomembranes of Recombinant Spider Silk Protein Support Cell Co-culture in an in Vitro Blood Vessel Wall Model". ACS Biomaterials Science & Engineering. 7 (7): 3332–3339. doi:10.1021/acsbiomaterials.1c00612. PMC 8290846. PMID 34169711.
- ^ Fischer, F. & Brander, J. (1960). "Eine Analyse der Gespinste der Kreuzspinne". Hoppe-Seyler's Zeitschrift für Physiologische Chemie. 320: 92–102. doi:10.1515/bchm2.1960.320.1.92. PMID 13699837.
- ^ Lucas, F.; Shaw, J. T. B. & Smith, S. G. (1960). "The Composition of Arthropod Silk Fibrons". Insect Chemistry. Symp. 3: 208–14.
- ^ Lucas, F.; Shaw, J. T. B. & Smith, S. G. (1960). "Comparative studies of fibroins.I. The amino acid composition of various fibroins and its significance in relation to their crystal structure and taxonomy". Journal of Molecular Biology. 2 (6): 339–49. doi:10.1016/S0022-2836(60)80045-9. PMID 13763962.
- ^ Xu, M. & Lewis, R. V. (1990). "Structure of a Protein Superfiber – Spider Dragline Silk". Proceedings of the National Academy of Sciences of the United States of America. 87 (18): 7120–24. Bibcode:1990PNAS...87.7120X. doi:10.1073/pnas.87.18.7120. PMC 54695. PMID 2402494.
- ^ Lucas, F. (1964). "Spiders and their silks". Discovery. 25: 20–26.
- ^ Vollrath, F. & Edmonds, D. T. (1989). "Modulation of the Mechanical-Properties of Spider Silk By Coating With Water". Nature. 340 (6231): 305–07. Bibcode:1989Natur.340..305V. doi:10.1038/340305a0. S2CID 4355740.
- ^ Vollrath, F.; Madsen, B. & Shao, Z. Z. (2001). "The effect of spinning conditions on the mechanics of a spider's dragline silk". Proceedings of the Royal Society B. 268 (1483): 2339–46. doi:10.1098/rspb.2001.1590. PMC 1088885. PMID 11703874.
- ^ Simmons, A.; Ray, E. & Jelinski, L. W. (1994). "Solid-State C-13 NMR of Nephila-Clavipes Dragline Silk Establishes Structure and Identity of Crystalline Regions". Macromolecules. 27 (18): 5235–37. Bibcode:1994MaMol..27.5235S. doi:10.1021/ma00096a060.
- ^ Shao, Z.; Vollrath, F.; Sirichaisit, J. & Young, R. J. (1999). "Analysis of spider silk in native and supercontracted states using Raman spectroscopy". Polymer. 40 (10): 2493–500. doi:10.1016/S0032-3861(98)00475-3.
- ^ Riekel, C.; Bränden, C; Craig, C; Ferrero, C; Heidelbach, F; Müller, M (1999). "Aspects of X-ray diffraction on single spider fibers". Int. J. Biol. Macromol. 24 (2–3): 179–86. doi:10.1016/S0141-8130(98)00084-1. PMID 10342763.
- ^ Knight, D. P.; Knight, M. M. & Vollrath, F. (2000). "Beta transition and stress-induced phase separation in the spinning of spider dragline silk". Int. J. Biol. Macromol. 27 (3): 205–10. doi:10.1016/S0141-8130(00)00124-0. PMID 10828366.
- ^ Riekel, C. & Vollrath, F. (2001). "Spider silk fibre extrusion: combined wide- and small-angle X- ray microdiffraction experiments". Int. J. Biol. Macromol. 29 (3): 203–10. doi:10.1016/S0141-8130(01)00166-0. PMID 11589973.
- ^ Gosline, J. M.; DeMont, M. E. & Denny, M. W. (1986). "The structure and properties of spider silk". Endeavour. 10: 37–43. doi:10.1016/0160-9327(86)90049-9.
- ^ Vollrath, F. & Porter, D. (2006). "Spider silk as an archetypal protein elastomer". Soft Matter. 2 (5): 377–85. Bibcode:2006SMat....2..377V. doi:10.1039/b600098n. PMID 32680251. S2CID 97234857.
- ^ Kerkam, K.; Viney, C.; Kaplan, D. & Lombardi, S. (1991). "Liquid Crystallinity of Natural Silk Secretions". Nature. 349 (6310): 596–98. Bibcode:1991Natur.349..596K. doi:10.1038/349596a0. S2CID 4348041.
- ^ Knight, D. P. & Vollrath, F. (1999). "Liquid crystals and flow elongation in a spider's silk production line". Proceedings of the Royal Society B. 266 (1418): 519–23. doi:10.1098/rspb.1999.0667. PMC 1689793.
- ^ Prince, J. T.; McGrath, K. P.; Digirolamo, C. M. & Kaplan, D. L. (1995). "Construction, Cloning, and Expression of Synthetic Genes Encoding Spider Dragline Silk". Biochemistry. 34 (34): 10879–85. doi:10.1021/bi00034a022. PMID 7662669.
- ^ Arcidiacono, S.; Mello, C.; Kaplan, D.; Cheley, S. & Bayley, H. (1998). "Purification and characterization of recombinant spider silk expressed in Escherichia coli". Applied Microbiology and Biotechnology. 49 (1): 31–38. doi:10.1007/s002530051133. PMID 9487707. S2CID 35267049.
- ^ Seidel, A.; Liivak, O. & Jelinski, L. W. (1998). "Artificial Spinning of Spider Silk". Macromolecules. 31 (19): 6733–36. Bibcode:1998MaMol..31.6733S. doi:10.1021/ma9808880.
- ^ Maev Kennedy (24 January 2012). "Spider silk cape goes on show at V&A". the Guardian.
- ^ Heimer, S. (1988). Wunderbare Welt der Spinnen. Urania. p. 14
- ^ Jackson, Robert R. (1974). "Effects of D-Amphetamine Sulfate and Diazepam on Thread Connection Fine Structure in a Spider's Web". Journal of Arachnology. 2 (1): 37–41. JSTOR 3704994.
- ^ "V&A · Golden spider silk". Victoria and Albert Museum. Retrieved 7 January 2022.
- ^ Leggett, Hadley (23 September 2009). "1 Million Spiders Make Golden Silk for Rare Cloth". Wired.
- ^ Allmeling, Christina; Jokuszies, Andreas; Reimers, Kerstin; Kall, Susanne; Vogt, Peter M. (2006). "Use of spider silk fibres as an innovative material in a biocompatible artificial nerve conduit". Journal of Cellular and Molecular Medicine. 10 (3): 770–77. doi:10.1111/j.1582-4934.2006.tb00436.x. PMC 3933158. PMID 16989736.
- ^ Berenbaum, May R., Field Notes – Spin Control, The Sciences, The New York Academy of Sciences, September/October 1995
- ^ Example of use of spider silk for telescopic rifle sights. Bonnier Corporation. 1955. Retrieved 24 August 2011.
- ^ Duarte F. J.; Taylor, T S; Black, A M; Davenport, W E; Varmette, P G (2011). "N-slit interferometer for secure free-space optical communications: 527 m intra interferometric path length". Journal of Optics. 13 (3): 5710. Bibcode:2011JOpt...13c5710D. doi:10.1088/2040-8978/13/3/035710. S2CID 6086533.
- ^ Osaki, Shigeyoshi (2012). "Spider Silk Violin Strings with a Unique Packing Structure Generate a Soft and Profound Timbre". Physical Review Letters. 108 (15): 154301. Bibcode:2012PhRvL.108o4301O. doi:10.1103/PhysRevLett.108.154301. PMID 22587257.
- ^ a b c d Service, Robert F. (18 October 2017). "Spinning spider silk into startup gold". Science Magazine, American Association for the Advancement of Science. Retrieved 26 November 2017.
- ^ Bonino, Mark J. "Material Properties of Spider Silk" (PDF).
- ^ Goodyer, Jason (5 July 2020). "Spider silk used to create lenses for imaging human tissue". BBC Science Focus.
- ^ Xia, Xiao-Xia; Qian, Zhi-Gang; Ki, Chang Seok; Park, Young Hwan; Kaplan, David L.; Lee, Sang Yup (2010). "Native-sized recombinant spider silk protein produced in metabolically engineered Escherichia coli results in a strong fiber". Proceedings of the National Academy of Sciences. 107 (32): 14059–63. Bibcode:2010PNAS..10714059X. doi:10.1073/pnas.1003366107. JSTOR 25708855. PMC 2922564. PMID 20660779.
- ^ "Draadkracht: spindoctors maken supersterk nepweb" [Wire strength: spin doctors make super strong fake cobweb] (in Dutch). KIJK. 21 April 2012. Retrieved 15 October 2014.
- ^ "Bolt Threads – Microsilk".
- ^ "Bolt Threads – B-silk protein".
- ^ "University of Notre Dame and Kraig Biocraft Laboratories Create Artificial Spider Silk Breakthrough" (Press release). Kraig Biocraft Laboratories. 29 September 2010. Retrieved 3 January 2012.
- ^ "Fraser Research Publicly Announced at Press Conference" (Press release). University of Notre Dame. 1 October 2010. Archived from the original on 10 October 2010. Retrieved 3 January 2012.
- ^ Kluge, Jonathan A.; Rabotyagova, Olena; Leisk, Gary G.; Kaplan, David L. (May 2008). "Spider silks and their applications". Trends in Biotechnology. 26 (5): 244–51. doi:10.1016/j.tibtech.2008.02.006. PMID 18367277.
- ^ Scheibel, Thomas (November 2004). "Spider silks: recombinant synthesis, assembly, spinning, and engineering of synthetic proteins". Microbial Cell Factories. 3 (1): 14. doi:10.1186/1475-2859-3-14. PMC 534800. PMID 15546497.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "Goldwin x Spiber Ski Jacket".
- ^ Bain, Marc (3 July 2016). "Synthetic spider silk could be the biggest technological advance in clothing since nylon". Quartz.
External links
- "The Silk Spinners", a BBC program about silk-producing animals
- Meadows, Robin (5 August 2014). "How Spiders Spin Silk". PLOS Biology. 12 (8): e1001922. doi:10.1371/journal.pbio.1001922. PMC 4122354. PMID 25093404.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Rejcek, Peter (11 April 2019). "The Tangled Web of Turning Spider Silk into a Super Material". Singularity Hub. Retrieved 24 April 2019.
- Archived at Ghostarchive and the Wayback Machine: Victoria and Albert Museum (29 July 2019). "How was it made? Golden spider silk". YouTube. Retrieved 8 August 2020.
- "Synthetic spider silk stronger and tougher than the real thing". New Atlas. 21 July 2021. Retrieved 21 July 2021.

![Schematic of the spider's orb web, structural modules, and spider silk structure.[37] On the left is shown a schematic drawing of an orb web. The red lines represent the dragline, radial line, and frame lines, the blue lines represent the spiral line, and the centre of the orb web is called the "hub". Sticky balls drawn in blue are made at equal intervals on the spiral line with viscous material secreted from the aggregate gland. Attachment cement secreted from the piriform gland is used to connect and fix different lines. Microscopically, the spider silk secondary structure is formed of spidroin and is said to have the structure shown on the right side. In the dragline and radial line, a crystalline β-sheet and an amorphous helical structure are interwoven. The large amount of β-spiral structure gives elastic properties to the capture part of the orb web. In the structural modules diagram, a microscopic structure of dragline and radial lines is shown, composed mainly of two proteins of MaSp1 and MaSp2, as shown in the upper central part. In the spiral line, there is no crystalline β-sheet region.](http://upload.wikimedia.org/wikipedia/commons/thumb/8/8a/0.Figure.png/900px-0.Figure.png)
