User:Graham Beards/viruses/What are viruses

Sir Peter Medawar (1915–1987) described a virus as "a piece of bad news wrapped in a protein coat".[1] With the exception of the bacteriophages, viruses had a well-deserved reputation for being nothing but the cause of diseases and death. The discovery of the abundance of viruses and their overwhelming presence in many ecosystems has led modern virologists to consider them in a new light.[2] It is estimated that there are 1031 viruses on Earth, most of them are bacteriophages, and most of them are in the oceans.[3] They are the most abundant species on Earth,[4] and they infect all types of organisms, from animals and plants to bacteria and archaea.[5] Since Dmitri Ivanovsky's 1892 article describing a non-bacterial pathogen infecting tobacco plants, and the discovery of the tobacco mosaic virus by Martinus Beijerinck in 1898,[6] about 5,000 viruses have been described in detail,[7] although there are millions of different types.[8] Viruses have existed since life first appeared on Earth although their origins are unclear.
Viruses are small infectious agents that can replicate only inside living cells. A single virus particle (known as a virion) consists of two or three parts: the genetic material made from either DNA or RNA, long molecules that carry genetic information; a protein coat that protects these genes; and in some cases an envelope of lipids that surrounds the protein coat when they are outside a cell. The average virus is about one one-hundredth the size of the average bacterium. Most viruses are too small to be seen directly with an optical microscope. It would take 30,000 to 750,000 viruses, side by side, to stretch to 1 centimetre (0.39 in).
Despite their size, the ability of viruses to cause disease and death has changed the course of human history. But although often feared, most viruses exist in peaceful coexistence with their hosts, and they play a key role in maintaining life on Earth.
Etymology[edit]
The word is from the Latin virus referring to poison and other noxious substances, first used in English in 1392.[9] Virulent, from Latin virulentus (poisonous), dates to 1400.[10] A meaning of "agent that causes infectious disease" is first recorded in 1728,[9] before the discovery of viruses by Dmitri Ivanovsky in 1892. The plural is viruses. The adjective viral dates to 1948.[11] The term virion (plural virions), which dates from 1959,[12] is also used to refer to a single, stable infective viral particle that is released from the cell and is fully capable of infecting other cells of the same type.[13]
Origins and evolution[edit]
Viruses are ancient. Studies at the molecular level have revealed relationships between viruses infecting organisms from each of the three domains of life, and viral proteins that pre-date the divergence of life and thus the last universal common ancestor.[14] This indicates that viruses emerged early in the evolution of life and existed before modern cells.[15] Viruses are found wherever there is life, [16] but they do not form fossils because they are much smaller than the grains of sedimentary rocks that fossilise plants and animals. The evolution of viruses has had to be traced by other methods. DNA sequencing has been the most powerful, and has provided unexpected insights.[17] Computers are used to measure viral relationships by comparing their DNA or RNA sequences. As would be expected, viruses of the same genus have much of their sequences in common and more distantly related viruses less in common, which is used to draw phylogenetic trees. The mutation rates for many viruses have been measured, allowing estimates to be made of when species of viruses diverged from common ancestors.[18]
There are three main hypotheses that try to explain the origins of viruses:[19][20]
- Regressive hypothesis
- Viruses may have once been small cells that parasitised larger cells. Over time, genes not required by their parasitism were lost. The bacteria rickettsia and chlamydia are living cells that, like viruses, can reproduce only inside host cells. They lend support to this hypothesis, as their dependence on parasitism is likely to have caused the loss of genes that enabled them to survive outside a cell. This is also called the degeneracy hypothesis,[21][22] or reduction hypothesis.[23]
- Cellular origin hypothesis
- Some viruses may have evolved from bits of DNA or RNA that "escaped" from the genes of a larger organism. The escaped DNA could have come from plasmids (pieces of naked DNA that can move between cells) or transposons (molecules of DNA that replicate and move around to different positions within the genes of the cell).[24] Once called "jumping genes", transposons are examples of mobile genetic elements and could be the origin of some viruses. They were discovered in maize by Barbara McClintock in 1950.[25] This is sometimes called the vagrancy hypothesis,[21][26] or the escape hypothesis.[23]
- Coevolution hypothesis
- This is also called the virus-first hypothesis[23] and proposes that viruses may have evolved from complex molecules of protein and nucleic acid at the same time as cells first appeared on Earth and would have been dependent on cellular life for billions of years. Viroids are molecules of RNA that are not classified as viruses because they lack a protein coat. However, they have characteristics that are common to several viruses and are often called subviral agents.[27] Viroids are important pathogens of plants.[28] They do not code for proteins but interact with the host cell and use the host machinery for their replication.[29] The hepatitis delta virus of humans has an RNA genome similar to viroids but has a protein coat derived from hepatitis B virus and cannot produce one of its own. It is, therefore, a defective virus and cannot replicate without the help of hepatitis B virus.[30] In similar manner, the sputnik virophage is dependent on mimivirus, which infects the protozoan Acanthamoeba castellanii.[31] These viruses that are dependent on the presence of other virus species in the host cell are called satellites and may represent evolutionary intermediates of viroids and viruses.[32][33]
There are problems with all of these hypotheses: the regressive hypothesis does not explain why even the smallest of cellular parasites do not resemble viruses in any way. The escape hypothesis does not explain the complex capsids and other structures on virus particles. The virus-first hypothesis contravened the definition of viruses in that they require host cells.[23] Viruses are now recognised as ancient and to have origins that pre-date the divergence of life into the three domains.[34] This discovery has led modern virologists to reconsider and re-evaluate these three classical hypotheses.[34]
The evidence for an ancestral world of RNA cells[35] and computer analysis of viral and host DNA sequences are giving a better understanding of the evolutionary relationships between different viruses and may help identify the ancestors of modern viruses. To date, such analyses have not proved which of these hypotheses is correct.[35] However, it seems unlikely that all currently known viruses have a common ancestor, and viruses have probably arisen numerous times in the past by one or more mechanisms.[36]
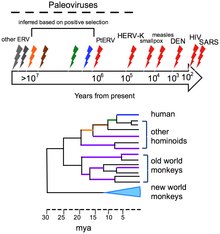
The human genome contains traces of ancient and extinct viruses that once infected hominids and primates, leaving copies of their DNA in the DNA of modern humans; about 31 different families of viruses, called human endogenous retroviruses, have been discovered.[37] Although retroviruses are RNA viruses (which have genes made from RNA not DNA), their reproduction involves a stage where their genes are translated into DNA and inserted into the host cell's DNA.[38] Most of these DNA copies stay in the host cell's genome permanently. A few enter the DNA of the reproductive cells and are passed down through generations of the host's offspring.[39] The last addition to the human genome is estimated to have occurred between 100,000 and 1,000,000 years ago.[37] The discovery of this ancient, once viral, DNA in the human genome has given birth to the science of paleovirology, which although still in its infancy has already provided insights into the co-evolution of humans and viruses and the development of human resistance to them. The study of these "virtual fossils" has become a valuable tool in the study of virus evolution.[37]
Viruses evolve following changes in their DNA (or RNA), some quite rapidly, and the best adapted mutants quickly outnumber their less fit counterparts. In this sense their evolution is Darwinian, just like that of their host organisms.[40] The way viruses reproduce in their host cells makes them particularly susceptible to the genetic changes that help to drive their evolution.[41] The RNA viruses are especially prone to mutations.[42] In host cells there are mechanisms for correcting mistakes when DNA replicates and these kick in whenever cells divide.[42] These important mechanisms prevent potentially lethal mutations from being passed on to offspring. But these mechanisms do not work for RNA and when an RNA virus replicates in its host cell, mutations are occasionally introduced in error, some of which are lethal. One virus particle can produce millions of progeny viruses in just one cycle of replication, therefore the production of a few "dud" viruses is not a problem. Most mutations are "silent" and do not result in any obvious changes to the progeny viruses, but others confer advantages that increase the fitness of the viruses in the environment. These could be changes to the virus particles that disguise them so they are not identified by the cells of the immune system or changes that make antiviral drugs less effective. Both of these changes occur frequently with HIV.[43]

Many viruses (for example, influenza A virus) can "shuffle" their genes with other viruses when two similar strains infect the same cell. This phenomenon is called genetic shift, and is often the cause of new and more virulent strains appearing. Other viruses change more slowly as mutations in their genes gradually accumulate over time, a process known as genetic drift.[45]
Through these mechanisms new viruses are constantly emerging and present a continuing challenge to attempts to control the diseases they cause.[46][47] Most species of viruses are now known to have common ancestors, and although the "virus first" hypothesis has yet to gain full acceptance, there is little doubt that the thousands of species of modern viruses have evolved from less numerous ancient ones.[48] The morbilliviruses, for example, are a group of closely related, but distinct viruses that infect a broad range of animals. The group includes measles virus, which infects humans and primates; canine distemper virus, which infects many animals including dogs, cats, bears, weasels and hyaenas; rinderpest, which infects cattle and buffalo; and other viruses of seals, porpoises and dolphins.[49] The herpes viruses are thought to have a common ancestor from 400 million years ago. During the Devonian period fish-like animals began to leave the oceans and colonise dry land where they became susceptible to ancestral herpes viruses. As these early amphibians evolved into different animal species, these viruses evolved within them. Today all vertebrates and some invertebrates have their own distinct types of herpes viruses and over 150 have been discovered.[50]
 The viruses – there are two of them – HIV-1 and HIV-2, originated in non-human primates and first infected humans in western equatorial Africa,[51] but HIV-1 rapidly spread across the world.[52] Shortly after HIV-1 was discovered in 1983 it was shown to be closely related to SIV, a retrovirus of monkeys and chimpanzees.[53] There are different strains of HIV-1 and HIV-2 and each evolved independently from primates. How these viruses entered the human population is not fully understood, but during the 1960s and 1970s there were large population shifts from rural to urban areas in Africa.[54] Analysis of the RNA from several strains of HIV-1 and HIV-2 has shown that these viruses evolved from SIV-related viruses during the first half of the 20th century.[55] This happened more the once and three groups of HIV-1 (called M, N and O) and two groups of HIV-2 (called A and B) emerged independently. This occurred around 1920 (O), 1930 (M) and N more recently. All of these groups of HIV had appeared by the 1960s and were circulating in central Africa.[56] The spread of HIV to the western world probably began in the 1960s when Haitians returned to their homeland from the Congo.[57] Apart from Africa, Haiti has the longest established HIV/AIDS epidemic and tourism during the 1960s probably aided the spread of the virus to the US, where in quietly circulated for over a decade before it was discovered.[58] Similarly, HIV-2 group A emerged in about 1940 and HIV-2 B in about 1945. But it is impossible to be precise, because the method of estimation used is statistical. Those dates represent the most likely years within a range of about ten years either side.[59] |
Organisms at the edge of life[edit]
Opinions differ on whether viruses are a form of life, or organic structures that interact with living organisms. They have been described as "organisms at the edge of life",[60] since they resemble organisms in that they possess genes and evolve by natural selection,[61] and reproduce by creating multiple copies of themselves through self-assembly. Although they have genes, they do not have a cellular structure, which is often seen as the basic unit of life. Viruses do not have their own metabolism, and require a host cell to make new products. They therefore cannot naturally reproduce outside a host cell[62] – although bacterial species such as rickettsia and chlamydia are considered living organisms despite the same limitation.[63][64] Accepted forms of life use cell division to reproduce, whereas viruses spontaneously assemble within cells. They differ from autonomous growth of crystals as they inherit genetic mutations while being subject to natural selection. Virus self-assembly within host cells has implications for the study of the origin of life, as it lends further credence to the hypothesis that life could have started as self-assembling organic molecules.[5]
Morphology[edit]


Viruses display a wide diversity of shapes and sizes, called morphologies. In general, viruses are much smaller than bacteria. Most viruses that have been studied have a diameter between 20 and 300 nanometres. Some filoviruses have a total length of up to 1400 nm; their diameters are only about 80 nm.[65] Most viruses cannot be seen with an optical microscope so scanning and transmission electron microscopes are used to visualise virions.[66] To increase the contrast between viruses and the background, electron-dense "stains" are used. These are solutions of salts of heavy metals, such as tungsten, that scatter the electrons from regions covered with the stain. When virions are coated with stain (positive staining), fine detail is obscured. Negative staining overcomes this problem by staining the background only.[67]

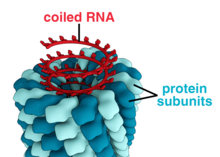
A complete virus particle, known as a virion, consists of nucleic acid surrounded by a protective coat of protein called a capsid. These are formed from identical protein subunits called capsomeres.[68] Viruses can have a lipid "envelope" derived from the host cell membrane. The capsid is made from proteins encoded by the viral genome and its shape serves as the basis for morphological distinction.[69][70] Virally coded protein subunits will self-assemble to form a capsid, in general requiring the presence of the virus genome. Complex viruses code for proteins that assist in the construction of their capsid. Proteins associated with nucleic acid are known as nucleoproteins, and the association of viral capsid proteins with viral nucleic acid is called a nucleocapsid. In general, there are four main morphological virus types:

- Helical
- These viruses are composed of a single type of capsomer stacked around a central axis to form a helical structure, which may have a central cavity, or hollow tube. This arrangement results in rod-shaped or filamentous virions: These can be short and highly rigid, or long and very flexible. The genetic material, in general, single-stranded RNA, but ssDNA in some cases, is bound into the protein helix by interactions between the negatively charged nucleic acid and positive charges on the protein. Overall, the length of a helical capsid is related to the length of the nucleic acid contained within it and the diameter is dependent on the size and arrangement of capsomers. The well-studied tobacco mosaic virus is an example of a helical virus.[71]
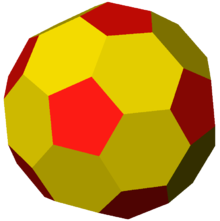
- Icosahedral
- Most animal viruses are icosahedral or near-spherical with icosahedral symmetry. A regular icosahedron is the optimum way of forming a closed shell from identical sub-units. The minimum number of identical capsomers required is twelve, each composed of five identical sub-units. Many viruses, such as rotavirus, have more than twelve capsomers and appear spherical but they retain this symmetry. Capsomers at the apices are surrounded by five other capsomers and are called pentons. Capsomers on the triangular faces are surrounded by six others and are called hexons.[72] Hexons are in essence flat and pentons, which form the 12 vertices, are curved. The same protein may act as the subunit of both the pentamers and hexamers or they may be composed of different proteins.
- Prolate
- This is an isosahedron elongated along the fivefold axis and is a common arrangement of the heads of bacteriophages. This structure is composed of a cylinder with a cap at either end.[73]
- Envelope
- Some species of virus envelop themselves in a modified form of one of the cell membranes, either the outer membrane surrounding an infected host cell or internal membranes such as nuclear membrane or endoplasmic reticulum, thus gaining an outer lipid bilayer known as a viral envelope. This membrane is studded with proteins coded for by the viral genome and host genome; the lipid membrane itself and any carbohydrates present originate entirely from the host. The influenza virus and HIV use this strategy. Most enveloped viruses are dependent on the envelope for their infectivity.[74]
- Complex
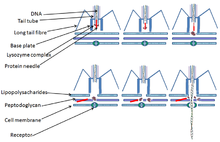
- These viruses possess a capsid that is neither purely helical nor purely icosahedral, and that may possess extra structures such as protein tails or a complex outer wall. Some bacteriophages, such as Enterobacteria phage T4, have a complex structure consisting of an icosahedral head bound to a helical tail, which may have a hexagonal base plate with protruding protein tail fibres. This tail structure acts like a molecular syringe, attaching to the bacterial host and then injecting the viral genome into the cell.[75]

Icosahedral viruses are assigned a triangulation number (T-number) to describe the relationship between the number of protein molecules that form pentagons or hexagons. The T-number idea was originally developed to explain the quasi-symmetry by Caspar and Klug in 1962.[76]
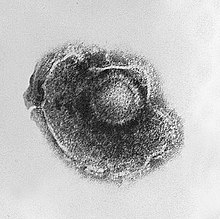
The poxviruses are large, complex viruses that have an unusual morphology. The viral genome is associated with proteins within a central disk structure known as a nucleoid. The nucleoid is surrounded by a membrane and two lateral bodies of unknown function. The virus has an outer envelope with a thick layer of protein studded over its surface. The whole virion is slightly pleiomorphic, ranging from ovoid to brick shape.[77] Mimivirus is the largest characterised virus, with a capsid diameter of 400 nm. Protein filaments measuring 100 nm project from the surface. The capsid appears hexagonal under an electron microscope, therefore the capsid is probably icosahedral.[78] In 2011, researchers discovered a larger virus on ocean floor of the coast of Las Cruces, Chile. Provisionally named Megavirus chilensis, it can be seen with a basic optical microscope. [79]
Some viruses that infect Archaea have complex structures that are unrelated to any other form of virus, with a wide variety of unusual shapes, ranging from spindle-shaped structures, to viruses that resemble hooked rods, teardrops or even bottles. Other archaeal viruses resemble the tailed bacteriophages, and can have multiple tail structures.[80]
Genomes[edit]
| Property | Parameters |
|---|---|
| Nucleic acid |
|
| Shape |
|
| Strandedness |
|
| Sense |
|
An enormous variety of genomic structures can be seen among viral species; as a group, they contain more structural genomic diversity than plants, animals, archaea, or bacteria. There are millions of different types of viruses,[8] although only about 5,000 of them have been described in detail.[81] A virus has either DNA or RNA genes and is called a DNA virus or a RNA virus, respectively. The vast majority of viruses have RNA genomes. Plant viruses tend to have single-stranded RNA genomes and bacteriophages tend to have double-stranded DNA genomes.[82]
Viral genomes are circular, as in the polyomaviruses, or linear, as in the adenoviruses. The type of nucleic acid is irrelevant to the shape of the genome. Among RNA viruses and certain DNA viruses, the genome is often divided up into separate parts, in which case it is called segmented. For RNA viruses, each segment often codes for only one protein and they are usually found together in one capsid. However, all segments are not required to be in the same virion for the virus to be infectious, as demonstrated by brome mosaic virus and several other plant viruses.[65]
A viral genome, irrespective of nucleic acid type, is almost always either single-stranded or double-stranded. Single-stranded genomes consist of an unpaired nucleic acid, analogous to one-half of a ladder split down the middle. Double-stranded genomes consist of two complementary paired nucleic acids, analogous to a ladder. The virus particles of some virus families, such as those belonging to the Hepadnaviridae, contain a genome that is partially double-stranded and partially single-stranded.[82]
For most viruses with RNA genomes and some with single-stranded DNA genomes, the single strands are said to be either positive-sense (called the plus-strand) or negative-sense (called the minus-strand), depending on whether or not they are complementary to the viral messenger RNA (mRNA). Positive-sense viral RNA is in the same sense as viral mRNA and thus at least a part of it can be immediately translated by the host cell. Negative-sense viral RNA is complementary to mRNA and thus must be converted to positive-sense RNA by an RNA-dependent RNA polymerase before translation. DNA nomenclature for viruses with single-sense genomic ssDNA is similar to RNA nomenclature, in that the coding strand for the viral mRNA is complementary to it (−), and the non-coding strand is a copy of it (+).[82] However, several types of ssDNA and ssRNA viruses have genomes that are ambisense in that transcription can occur off both strands in a double-stranded replicative intermediate. Examples include geminiviruses, which are ssDNA plant viruses and arenaviruses, which are ssRNA viruses of animals.[83]
Genome size varies greatly between species. The smallest viral genomes – the ssDNA circoviruses, family Circoviridae – code for only two proteins and have a genome size of only 2 kilobases; the largest – mimiviruses – have genome sizes of over 1.2 megabases and code for over one thousand proteins.[84] In general, RNA viruses have smaller genome sizes than DNA viruses because of a higher error-rate when replicating, and have a maximum upper size limit.[85] Beyond this limit, errors in the genome when replicating render the virus useless or uncompetitive. To compensate for this, RNA viruses often have segmented genomes – the genome is split into smaller molecules – thus reducing the chance that an error in a single-component genome will incapacitate the entire genome. In contrast, DNA viruses generally have larger genomes because of the high fidelity of their replication enzymes.[86] Single-strand DNA viruses are an exception to this rule, however, as mutation rates for these genomes can approach the extreme of the ssRNA virus case.[87]

Viruses undergo genetic change by several mechanisms. These include a process called genetic drift where individual bases in the DNA or RNA mutate to other bases. Most of these point mutations are "silent" – they do not change the protein that the gene encodes – but others can confer evolutionary advantages such as resistance to antiviral drugs.[88] Antigenic shift occurs when there is a major change in the genome of the virus. This can be a result of recombination or reassortment. When this happens with influenza viruses, pandemics might result.[89] RNA viruses often exist as quasispecies or swarms of viruses of the same species but with slightly different genome nucleoside sequences. Such quasispecies are a prime target for natural selection.[90]
Segmented genomes confer evolutionary advantages; different strains of a virus with a segmented genome can shuffle and combine genes and produce progeny viruses or (offspring) that have unique characteristics. This is called reassortment or viral sex.[91]
Genetic recombination is the process by which a strand of DNA is broken and then joined to the end of a different DNA molecule. This can occur when viruses infect cells simultaneously and studies of viral evolution have shown that recombination has been rampant in the species studied.[92] Recombination is common to both RNA and DNA viruses.[93][94]
Replication cycle[edit]

Proteins are essential to life. Cells produce new protein molecules from amino acid building blocks based on information coded in DNA. Each type of protein is a specialist that only performs one function, so if a cell needs to do something new, it must make a new protein. Viruses force the cell to make new proteins that the cell does not need, but are needed for the virus to reproduce. Protein synthesis basically consists of two major steps: transcription and translation.
Transcription is the process where information in DNA, called the genetic code, is used to produce RNA copies called messenger RNA (mRNA). These migrate through the cell and carry the code to ribosomes where it is used to make proteins. This is called translation because the protein's amino acid structure is determined by the mRNA's code. Information is hence translated from the language of nucleic acids to the language of amino acids. Some RNA genes of viruses function directly as mRNA without further modification. For this reason, these viruses are called positive-sense RNA viruses.[95] In other RNA viruses, the RNA is a complementary copy of mRNA and these viruses rely on the cell's or their own enzyme to make mRNA. These are called negative-sense RNA viruses. In viruses made from DNA, the method of mRNA production is similar to that of the cell. The species of viruses called retroviruses behave completely differently: they have RNA, but inside the host cell a DNA copy of their RNA is made with the help of the enzyme reverse transcriptase. This DNA is then incorporated into the host's, and copied into mRNA by the cell's normal pathways.[96] Viral populations do not grow through cell division, because they are acellular. Instead, they use the machinery and metabolism of a host cell to produce multiple copies of themselves, and they assemble in the cell.
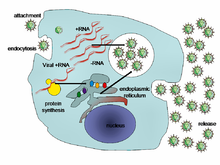
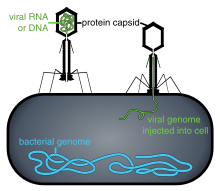
The life cycle of viruses differs greatly between species but there are six basic stages in the life cycle of viruses:[97]
- Attachment is a specific binding between viral capsid proteins and specific receptors on the host cellular surface. This specificity determines the host range of a virus. For example, HIV infects a limited range of human leucocytes. This is because its surface protein, gp120, specifically interacts with the CD4 molecule – a chemokine receptor – which is most commonly found on the surface of CD4+ T-Cells. This mechanism has evolved to favour those viruses that infect only cells in which they are capable of replication. Attachment to the receptor can induce the viral envelope protein to undergo changes that results in the fusion of viral and cellular membranes, or changes of non-enveloped virus surface proteins that allow the virus to enter.
- Penetration follows attachment: Virions enter the host cell through receptor-mediated endocytosis or membrane fusion. This is often called viral entry. The infection of plant and fungal cells is different from that of animal cells. Plants have a rigid cell wall made of cellulose, and fungi one of chitin, so most viruses can get inside these cells only after trauma to the cell wall.[98] However, nearly all plant viruses (such as tobacco mosaic virus) can also move directly from cell to cell, in the form of single-stranded nucleoprotein complexes, through pores called plasmodesmata.[99] Bacteria, like plants, have strong cell walls that a virus must breach to infect the cell. However, given that bacterial cell walls are much less thick than plant cell walls due to their much smaller size, some viruses have evolved mechanisms that inject their genome into the bacterial cell across the cell wall, while the viral capsid remains outside.[100]
- Uncoating is a process in which the viral capsid is removed: This may be by degradation by viral enzymes or host enzymes or by simple dissociation; the end-result is the releasing of the viral genomic nucleic acid.
- Replication of viruses involves primarily multiplication of the genome. Replication involves synthesis of viral messenger RNA (mRNA) from "early" genes (with exceptions for positive sense RNA viruses), viral protein synthesis, possible assembly of viral proteins, then viral genome replication mediated by early or regulatory protein expression. This may be followed, for complex viruses with larger genomes, by one or more further rounds of mRNA synthesis: "late" gene expression is, in general, of structural or virion proteins.
- Assembly - Following the structure-mediated self-assembly of the virus particles, some modification of the proteins often occurs. In viruses such as HIV, this modification (sometimes called maturation) occurs after the virus has been released from the host cell.[101]
- Release- Viruses can be released from the host cell by lysis, a process that kills the cell by bursting its membrane and cell wall if present: This is a feature of many bacterial and some animal viruses. Some viruses undergo a lysogenic cycle where the viral genome is incorporated by genetic recombination into a specific place in the host's chromosome. The viral genome is then known as a "provirus" or, in the case of bacteriophages a "prophage".[102] Whenever the host divides, the viral genome is also replicated. The viral genome is mostly silent within the host; however, at some point, the provirus or prophage may give rise to active virus, which may lyse the host cells.[103] Enveloped viruses (e.g., HIV) typically are released from the host cell by budding. During this process the virus acquires its envelope, which is a modified piece of the host's plasma or other, internal membrane.[104]
The genetic material within virus particles, and the method by which the material is replicated, varies considerably between different types of viruses.
- DNA viruses
- The genome replication of most DNA viruses takes place in the cell's nucleus. If the cell has the appropriate receptor on its surface, these viruses enter the cell sometimes by direct fusion with the cell membrane (e.g., herpesviruses) or – more usually – by receptor-mediated endocytosis. Most DNA viruses are entirely dependent on the host cell's DNA and RNA synthesising machinery, and RNA processing machinery; however, viruses with larger genomes may encode much of this machinery themselves. In eukaryotes the viral genome must cross the cell's nuclear membrane to access this machinery, while in bacteria it need only enter the cell.[105]
- RNA viruses
- Replication usually takes place in the cytoplasm. RNA viruses can be placed into four different groups depending on their modes of replication. The polarity (whether or not it can be used directly by ribosomes to make proteins) of single-stranded RNA viruses largely determines the replicative mechanism; the other major criterion is whether the genetic material is single-stranded or double-stranded. All RNA viruses use their own RNA replicase enzymes to create copies of their genomes.[106]
- Reverse transcribing viruses
- These have ssRNA (Retroviridae, Metaviridae, Pseudoviridae) or dsDNA (Caulimoviridae, and Hepadnaviridae) in their particles. Reverse transcribing viruses with RNA genomes (retroviruses), use a DNA intermediate to replicate, whereas those with DNA genomes (pararetroviruses) use an RNA intermediate during genome replication. Both types use a reverse transcriptase, or RNA-dependent DNA polymerase enzyme, to carry out the nucleic acid conversion. Retroviruses integrate the DNA produced by reverse transcription into the host genome as a provirus as a part of the replication process; pararetroviruses do not, although integrated genome copies of especially plant pararetroviruses can give rise to infectious virus.[107] They are susceptible to antiviral drugs that inhibit the reverse transcriptase enzyme, e.g. zidovudine and lamivudine. An example of the first type is HIV, which is a retrovirus. Examples of the second type are the Hepadnaviridae, which includes Hepatitis B virus.[108]
Some viruses, called satellites, can replicate only within cells that have already been infected by another virus. These include hepatitis D virus, which grows only in the presence of the hepatitis B virus,[109] adeno-associated viruses,[110] and the virophages of the mimivirus.[31]
Effects on the host cell[edit]
The range of structural and biochemical effects that viruses have on the host cell is extensive.[111] These are called cytopathic effects.[112] Most virus infections eventually result in the death of the host cell. The causes of death include cell lysis, alterations to the cell's surface membrane and apoptosis.[113] Often cell death is caused by cessation of its normal activities because of suppression by virus-specific proteins, not all of which are components of the virus particle.[114]
Some viruses cause no apparent changes to the infected cell. Cells in which the virus is latent and inactive show few signs of infection and often function normally.[115] This causes persistent infections and the virus is often dormant for many months or years. This is often the case with herpes viruses.[116][117] Some viruses, such as Epstein-Barr virus, can cause cells to proliferate without causing malignancy,[118] while others, such as papillomaviruses, are established causes of cancer.[119]
Host range[edit]
Viruses are by far the most abundant biological entities on Earth and they outnumber all the others put together.[120] They infect all types of cellular life including animals, plants, bacteria and fungi.[121] However, different types of viruses can infect only a limited range of hosts and many are species-specific. Some, such as smallpox virus for example, can infect only one species – in this case humans,[122] and are said to have a narrow host range. Other viruses, such as rabies virus, can infect different species of mammals and are said to have a broad range.[123] The viruses that infect plants are harmless to animals, and most viruses that infect other animals are harmless to humans.[124] The host range of some bacteriophages is limited to a single strain of bacteria and they can be used to trace the source of outbreaks of infections by a method called phage typing.[125]
Classification[edit]
Classification seeks to describe the diversity of viruses by naming and grouping them on the basis of similarities. In 1962, André Lwoff, Robert Horne, and Paul Tournier were the first to develop a means of virus classification, based on the Linnaean hierarchical system.[126] This system bases classification on phylum, class, order, family, genus, and species. Viruses were grouped according to their shared properties (not those of their hosts) and the type of nucleic acid forming their genomes.[127] Later the International Committee on Taxonomy of Viruses was formed. However, viruses are not classified on the basis of phylum or class, as their small genome size and high rate of mutation makes it difficult to determine their ancestry beyond Order. As such, the Baltimore Classification is used to supplement the more traditional hierarchy.
ICTV classification[edit]
The International Committee on Taxonomy of Viruses (ICTV) developed the current classification system and wrote guidelines that put a greater weight on certain virus properties to maintain family uniformity. A unified taxonomy (a universal system for classifying viruses) has been established. The 7th lCTV Report formalised for the first time the concept of the virus species as the lowest taxon (group) in a branching hierarchy of viral taxa.[128] However, at present only a small part of the total diversity of viruses has been studied, with analyses of samples from humans finding that about 20% of the virus sequences recovered have not been seen before, and samples from the environment, such as from seawater and ocean sediments, finding that the large majority of sequences are completely novel.[129]
The general taxonomic structure is as follows:
In the current (2011) ICTV taxonomy, six orders have been established, the Caudovirales, Herpesvirales, Mononegavirales, Nidovirales, Picornavirales and Tymovirales. A seventh order Ligamenvirales has also been proposed. The committee does not formally distinguish between subspecies, strains, and isolates. In total there are 6 orders, 87 families, 19 subfamilies, 349 genera, about 2,284 species and over 3,000 types yet unclassified.[130][131][132]
Baltimore classification[edit]

The Nobel Prize-winning biologist David Baltimore devised the Baltimore classification system.[133][134] The ICTV classification system is used in conjunction with the Baltimore classification system in modern virus classification.[135][136][137]
The Baltimore classification of viruses is based on the mechanism of mRNA production. Viruses must generate mRNAs from their genomes to produce proteins and replicate themselves, but different mechanisms are used to achieve this in each virus family. Viral genomes may be single-stranded (ss) or double-stranded (ds), RNA or DNA, and may or may not use reverse transcriptase (RT). In addition, ssRNA viruses may be either sense (+) or antisense (−). This classification places viruses into seven groups:
- I: dsDNA viruses (e.g. Adenoviruses, Herpesviruses, Poxviruses)
- II: ssDNA viruses (+)sense DNA (e.g. Parvoviruses)
- III: dsRNA viruses (e.g. Reoviruses)
- IV: (+)ssRNA viruses (+)sense RNA (e.g. Picornaviruses, Togaviruses)
- V: (−)ssRNA viruses (−)sense RNA (e.g. Orthomyxoviruses, Rhabdoviruses)
- VI: ssRNA-RT viruses (+)sense RNA with DNA intermediate in life-cycle (e.g. Retroviruses)
- VII: dsDNA-RT viruses (e.g. Hepadnaviruses)
As an example of viral classification, the chicken pox virus, varicella zoster (VZV), belongs to the order Herpesvirales, family Herpesviridae, subfamily Alphaherpesvirinae, and genus Varicellovirus. VZV is in Group I of the Baltimore Classification because it is a dsDNA virus that does not use reverse transcriptase.
- ^ Quoted in:Eskild Peterson; Ryan, Kenneth J.; Nafees Ahmad (2010). Sherris Medical Microbiology, Fifth Edition. McGraw-Hill Medical. p. 101. ISBN 0-07-160402-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Thurber RV (2009). "Current insights into phage biodiversity and biogeography". Curr. Opin. Microbiol. 12 (5): 582–7. doi:10.1016/j.mib.2009.08.008. PMID 19811946.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Breitbart M, Rohwer F (2005). "Here a virus, there a virus, everywhere the same
virus?". Trends in Microbiology. 13 (6): 278–84. doi:10.1016/j.tim.2005.04.003. PMID 15936660.
{{cite journal}}: Unknown parameter|month=ignored (help); line feed character in|title=at position 49 (help) - ^ Zimmer, p. 43
- ^ a b Koonin EV, Senkevich TG, Dolja VV. The ancient Virus World and evolution of cells. Biol. Direct. 2006;1:29. doi:10.1186/1745-6150-1-29. PMID 16984643.
- ^ Carter, P. 4
- ^ Dimmock p. 49
- ^ a b Breitbart M, Rohwer F. Here a virus, there a virus, everywhere the same virus?. Trends Microbiol. 2005;13(6):278–84. doi:10.1016/j.tim.2005.04.003. PMID 15936660.
- ^ a b Harper D. The Online Etymology Dictionary. virus; 2011 [Retrieved 23 December 2011].
- ^ Harper D. The Online Etymology Dictionary. virulent; 2011 [Retrieved 23 December 2011].
- ^ Harper D. The Online Etymology Dictionary. viral; 2011 [Retrieved 23 December 2011].
- ^ Harper D. The Online Etymology Dictionary. virion; 2011 [Retrieved 24 December 2011].
- ^ Casjens S. In: Mahy BWJ and Van Regenmortel MHV. Desk Encyclopedia of General Virology. Boston: Academic Press; 2010. ISBN 0-12-375146-2. p. 167.
- ^ Mahy,(a) p. 25
- ^ Mahy, (a) p. 26
- ^ Iyer LM, Balaji S, Koonin EV, Aravind L. Evolutionary genomics of nucleo-cytoplasmic large DNA viruses. Virus Res.. 2006;117(1):156–84. doi:10.1016/j.virusres.2006.01.009. PMID 16494962.
- ^ Mahy, (a) pp. 66–79
- ^ Lam TT, Hon CC, Tang JW (2010). "Use of phylogenetics in the molecular epidemiology and evolutionary studies of viral infections". Critical Reviews in Clinical Laboratory Sciences. 47 (1): 5–49. doi:10.3109/10408361003633318. PMID 20367503.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Shors pp. 14–16
- ^ Collier pp. 11–21
- ^ a b Dimmock p. 16
- ^ Collier p. 11
- ^ a b c d Mahy WJ & Van Regenmortel MHV (eds). Desk Encyclopedia of General Virology. Oxford: Academic Press; 2009. ISBN 0-12-375146-2. p. 24.
- ^ Shors p. 574
- ^ The origin and behavior of mutable loci in maize. Proc Natl Acad Sci U S A.. 1950;36(6):344–55. doi:10.1073/pnas.36.6.344. PMID 15430309.
- ^ Collier pp. 11–12
- ^ Dimmock p. 55
- ^ Shors 551–3
- ^ Tsagris EM, de Alba AE, Gozmanova M, Kalantidis K. Viroids. Cell. Microbiol.. 2008;10(11):2168. doi:10.1111/j.1462-5822.2008.01231.x. PMID 18764915.
- ^ Shors p. 492–3
- ^ a b La Scola B, Desnues C, Pagnier I, Robert C, Barrassi L, Fournous G, Merchat M, Suzan-Monti M, Forterre P, Koonin E, Raoult D. The virophage as a unique parasite of the giant mimivirus. Nature. 2008;455(7209):100–4. doi:10.1038/nature07218. PMID 18690211. Cite error: The named reference "pmid18690211" was defined multiple times with different content (see the help page).
- ^ Collier p. 777
- ^ Dimmock p. 55–7
- ^ a b Mahy WJ & Van Regenmortel MHV (eds). Desk Encyclopedia of General Virology. Oxford: Academic Press; 2009. ISBN 0-12-375146-2. p. 28.
- ^ a b Mahy WJ & Van Regenmortel MHV (eds). Desk Encyclopedia of General Virology. Oxford: Academic Press; 2009. ISBN 0-12-375146-2. p. 26.
- ^ Dimmock pp. 15–16
- ^ a b c d Emerman M, Malik HS (2010). Virgin, Skip W. (ed.). "Paleovirology—modern consequences of ancient viruses". PLoS Biology. 8 (2): e1000301. doi:10.1371/journal.pbio.1000301. PMC 2817711. PMID 20161719.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: unflagged free DOI (link) - ^ Bannert N, Kurth R (2006). "The evolutionary dynamics of human endogenous retroviral families". Annual Review of Genomics and Human Genetics. 7: 149–73. doi:10.1146/annurev.genom.7.080505.115700. PMID 16722807.
- ^ Jern P, Coffin JM (2008). "Effects of retroviruses on host genome function". Annual Review of Genetics. 42: 709–32. doi:10.1146/annurev.genet.42.110807.091501. PMID 18694346.
- ^ Dimmock, p. 273
- ^ Dimmock, p. 272
- ^ a b Domingo E, Escarmís C, Sevilla N, Moya A, Elena SF, Quer J, Novella IS, Holland JJ (1996). "Basic concepts in RNA virus evolution". The FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 10 (8): 859–64. PMID 8666162.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Boutwell CL, Rolland MM, Herbeck JT, Mullins JI, Allen TM (2010). "Viral evolution and escape during acute HIV-1 infection". The Journal of Infectious Diseases. 202 Suppl 2: S309–14. doi:10.1086/655653. PMC 2945609. PMID 20846038.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Barrett, p. 24
- ^ Chen J, Deng YM (2009). "Influenza virus antigenic variation, host antibody production and new approach to control epidemics". Virology Journal. 6: 30. doi:10.1186/1743-422X-6-30. PMC 2666653. PMID 19284639.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Fraile A, García-Arenal F (2010). "The coevolution of plants and viruses: resistance and
pathogenicity". Advances in Virus Research. 76: 1–32. doi:10.1016/S0065-3527(10)76001-2. PMID 20965070.
{{cite journal}}: line feed character in|title=at position 54 (help) - ^ Tang JW, Shetty N, Lam TT, Hon KL (2010). "Emerging, novel, and known influenza virus infections in humans". Infectious Disease Clinics of North America. 24 (3): 603–17. doi:10.1016/j.idc.2010.04.001. PMID 20674794.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Mahy, (a) pp. 70–80
- ^ Barrett, p. 16
- ^ Crawford (2011) p. 66–67
- ^ Mahy (b) pp. 340–343
- ^ Van Heuverswyn F, Peeters M (2007). "The origins of HIV and implications for the global epidemic". Current Infectious Disease Reports. 9 (4): 338–46. doi:10.1007/s11908-007-0052-x. PMID 17618555.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Paiardini M, Pandrea I, Apetrei C, Silvestri G (2009). "Lessons learned from the natural hosts of HIV-related viruses". Annual Review of Medicine. 60: 485–95. doi:10.1146/annurev.med.60.041807.123753. PMID 19630581.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Weeks, p. 19
- ^ Sharp PM, Hahn BH (2010). "The evolution of HIV-1 and the origin of AIDS". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 365 (1552): 2487–94. doi:10.1098/rstb.2010.0031. PMC 2935100. PMID 20643738.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Zimmer, p. 59
- ^ Zimmer, p. 60
- ^ Gilbert, MT.; Rambaut, A.; Wlasiuk, G.; Spira, TJ.; Pitchenik, AE.; Worobey, M. (2007). "The emergence of HIV/AIDS in the Americas and beyond". Proc Natl Acad Sci U S A. 104 (47): 18566–70. doi:10.1073/pnas.0705329104. PMC 2141817. PMID 17978186.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Mahy, (b) p. 343
- ^ Rybicki, EP. The classification of organisms at the edge of life, or problems with virus systematics. S Afr J Sci. 1990;86:182–186.
- ^ Holmes EC. Viral evolution in the genomic age. PLoS Biol.. 2007;5(10):e278. doi:10.1371/journal.pbio.0050278. PMID 17914905.
- ^ Wimmer E, Mueller S, Tumpey TM, Taubenberger JK. Synthetic viruses: a new opportunity to understand and prevent viral disease. Nature Biotechnology. 2009;27(12):1163–72. doi:10.1038/nbt.1593. PMID 20010599.
- ^ Horn M. Chlamydiae as symbionts in eukaryotes. Annual Review of Microbiology. 2008;62:113–31. doi:10.1146/annurev.micro.62.081307.162818. PMID 18473699.
- ^ Ammerman NC, Beier-Sexton M, Azad AF. Laboratory maintenance of Rickettsia rickettsii. Current Protocols in Microbiology. 2008;Chapter 3:Unit 3A.5. doi:10.1002/9780471729259.mc03a05s11. PMID 19016440.
- ^ a b Collier pp. 33–55
- ^ Collier pp. 33–37
- ^ Kiselev NA, Sherman MB, Tsuprun VL. Negative staining of proteins. Electron Microsc. Rev.. 1990;3(1):43–72. doi:10.1016/0892-0354(90)90013-I. PMID 1715774.
- ^ Collier p. 40
- ^ Caspar DL, Klug A. Physical principles in the construction of regular viruses. Cold Spring Harb. Symp. Quant. Biol.. 1962;27:1–24. PMID 14019094.
- ^ Crick FH, Watson JD. Structure of small viruses. Nature. 1956;177(4506):473–5. doi:10.1038/177473a0. PMID 13309339.
- ^ Collier p. 37
- ^ Collier pp. 40, 42
- ^ Casens, S.. Desk Encyclopedia of General Virology. Boston: Academic Press; 2009. ISBN 0-12-375146-2. p. 167–174.
- ^ Collier pp. 42–43
- ^ Rossmann MG, Mesyanzhinov VV, Arisaka F, Leiman PG. The bacteriophage T4 DNA injection machine. Curr. Opin. Struct. Biol.. 2004;14(2):171–80. doi:10.1016/j.sbi.2004.02.001. PMID 15093831.
- ^ Caspar, D. L. D. and Klug, A. (1962). "Physical Principles in the Construction of Regular Viruses". Cold Spring Harbor Symp. Quant. Biol. 27: 1–24. PMID 14019094.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Long GW, Nobel J, Murphy FA, Herrmann KL, Lourie B. Experience with electron microscopy in the differential diagnosis of smallpox. Appl Microbiol. 1970;20(3):497–504. PMID 4322005.
- ^ Suzan-Monti M, La Scola B, Raoult D. Genomic and evolutionary aspects of Mimivirus. Virus Research. 2006;117(1):145–155. doi:10.1016/j.virusres.2005.07.011. PMID 16181700.
- ^ World’s biggest virus discovered in ocean depths near Chile [Retrieved 12 October 2011].
- ^ Prangishvili D, Forterre P, Garrett RA. Viruses of the Archaea: a unifying view. Nat. Rev. Microbiol.. 2006;4(11):837–48. doi:10.1038/nrmicro1527. PMID 17041631.
- ^ Dimmock p. 49
- ^ a b c Collier pp. 96–99
- ^ Saunders, Venetia A.; Carter, John. Virology: principles and applications. Chichester: John Wiley & Sons; 2007. ISBN 0-470-02387-2. p. 72.
- ^ Van Etten JL, Lane LC, Dunigan DD. DNA viruses: the really big ones (giruses). Annual Review of Microbiology. 2010;64:83–99. doi:10.1146/annurev.micro.112408.134338. PMID 20690825.
- ^ Sanjuán R,
Nebot MR, Chirico N, Mansky LM, Belshaw R (2010). "Viral mutation rates". Journal of Virology. 84 (19): 9733–48. doi:10.1128/JVI.00694-10. PMC 2937809. PMID 20660197.
{{cite journal}}: Unknown parameter|month=ignored (help); line feed character in|author=at position 11 (help)CS1 maint: multiple names: authors list (link) - ^ Pressing J, Reanney DC. Divided genomes and intrinsic noise. J Mol Evol. 1984;20(2):135–46. doi:10.1007/BF02257374. PMID 6433032.
- ^ Duffy S, Holmes EC. Validation of high rates of nucleotide substitution in geminiviruses: phylogenetic evidence from East African cassava mosaic viruses. The Journal of General Virology. 2009;90(Pt 6):1539–47. doi:10.1099/vir.0.009266-0. PMID 19264617.
- ^ Pan XP, Li LJ, Du WB, Li MW, Cao HC, Sheng JF. Differences of YMDD mutational patterns, precore/core promoter mutations, serum HBV DNA levels in lamivudine-resistant hepatitis B genotypes B and C. J. Viral Hepat.. 2007;14(11):767–74. doi:10.1111/j.1365-2893.2007.00869.x. PMID 17927612.
- ^ Hampson AW, Mackenzie JS. The influenza viruses. Med. J. Aust.. 2006;185(10 Suppl):S39–43. PMID 17115950.
- ^ Metzner KJ. Detection and significance of minority quasispecies of drug-resistant HIV-1. J HIV Ther. 2006;11(4):74–81. PMID 17578210.
- ^ Goudsmit, Jaap. Viral Sex. Oxford Univ Press, 1998.ISBN 978-0-19-512496-5 ISBN 0-19-512496-0
- ^ Worobey M, Holmes EC. Evolutionary aspects of recombination in RNA viruses. J. Gen. Virol.. 1999;80 ( Pt 10):2535–43. PMID 10573145.
- ^ Lukashev AN. Role of recombination in evolution of enteroviruses. Rev. Med. Virol.. 2005;15(3):157–67. doi:10.1002/rmv.457. PMID 15578739.
- ^ Umene K. Mechanism and application of genetic recombination in herpesviruses. Rev. Med. Virol.. 1999;9(3):171–82. doi:10.1002/(SICI)1099-1654(199907/09)9:3<171::AID-RMV243>3.0.CO;2-A. PMID 10479778.
- ^ Collier pp. 75–82
- ^ Shors pp. 248–250
- ^ Collier pp. 75–91
- ^ Dimmock p. 70
- ^ Boevink P, Oparka KJ. Virus-host interactions during movement processes. Plant Physiol.. 2005;138(4):1815–21. doi:10.1104/pp.105.066761. PMID 16172094. PMC 1183373.
- ^ Dimmock p. 71
- ^ Barman S, Ali A, Hui EK, Adhikary L, Nayak DP. Transport of viral proteins to the apical membranes and interaction of matrix protein with glycoproteins in the assembly of influenza viruses. Virus Res.. 2001;77(1):61–9. doi:10.1016/S0168-1702(01)00266-0. PMID 11451488.
- ^ Shors pp. 60, 597
- ^ Dimmock, Chapter 15, Mechanisms in virus latentcy, pp.243–259
- ^ Dimmock 185–187
- ^ Shors p. 54; Collier p. 78
- ^ Collier p. 79
- ^ Staginnus C, Richert-Pöggeler KR. Endogenous pararetroviruses: two-faced travelers in the plant genome. Trends in Plant Science. 2006;11(10):485–91. doi:10.1016/j.tplants.2006.08.008. PMID 16949329.
- ^ Collier pp. 88–89
- ^ Makino S; Chang MF; Shieh CK; et al. (1987). "Molecular cloning and sequencing of a human hepatitis delta (delta) virus RNA". Nature. 329 (6137): 343–6. doi:10.1038/329343a0. PMID 3627276.
{{cite journal}}: Unknown parameter|author-separator=ignored (help) - ^ Gonçalves, M (2005). "Adeno-associated virus: from defective virus to effective vector". Virology Journal. 2 (1): 43–60. doi:10.1186/1743-422X-2-43. PMC 1131931. PMID 15877812.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Collier pp. 115–146
- ^ Collier p. 115
- ^ Roulston A, Marcellus RC, Branton PE. Viruses and apoptosis. Annu. Rev. Microbiol.. 1999;53:577–628. doi:10.1146/annurev.micro.53.1.577. PMID 10547702.
- ^ Alwine JC. Modulation of host cell stress responses by human cytomegalovirus. Curr. Top. Microbiol. Immunol.. 2008;325:263–79. doi:10.1007/978-3-540-77349-8_15. PMID 18637511.
- ^ Sinclair J. Human cytomegalovirus: Latency and reactivation in the myeloid lineage. J. Clin. Virol.. 2008;41(3):180–5. doi:10.1016/j.jcv.2007.11.014. PMID 18164651.
- ^ Jordan MC, Jordan GW, Stevens JG, Miller G. Latent herpesviruses of humans. Ann. Intern. Med.. 1984;100(6):866–80. PMID 6326635.
- ^ Sissons JG, Bain M, Wills MR. Latency and reactivation of human cytomegalovirus. J. Infect.. 2002;44(2):73–7. doi:10.1053/jinf.2001.0948. PMID 12076064.
- ^ Barozzi P, Potenza L, Riva G, Vallerini D, Quadrelli C, Bosco R, Forghieri F, Torelli G, Luppi M. B cells and herpesviruses: a model of lymphoproliferation. Autoimmun Rev. 2007;7(2):132–6. doi:10.1016/j.autrev.2007.02.018. PMID 18035323.
- ^ Subramanya D, Grivas PD. HPV and cervical cancer: updates on an established relationship. Postgrad Med. 2008;120(4):7–13. doi:10.3810/pgm.2008.11.1928. PMID 19020360.
- ^ Crawford, Dorothy H.. Viruses: A Very Short Introduction. Oxford University Press, USA; 2011. ISBN 0-19-957485-5. p. 16.
- ^ Dimmock p. 49
- ^ Shors p. 388
- ^ Shors p. 353
- ^ Dimmock p. 272
- ^ Baggesen DL, Sørensen G, Nielsen EM, Wegener HC. Phage typing of Salmonella Typhimurium – is it still a useful tool for surveillance and outbreak investigation?. Eurosurveillance. 2010;15(4):19471. PMID 20122382.
- ^ Lwoff A, Horne RW, Tournier P. A virus system. C. R. Hebd. Seances Acad. Sci.. 1962;254:4225–7. French. PMID 14467544.
- ^ Lwoff A, Horne R, Tournier P. A system of viruses. Cold Spring Harb. Symp. Quant. Biol.. 1962;27:51–5. PMID 13931895.
- ^ Knipe p. 27
- As defined therein, "a virus species is a polythetic class of viruses that constitute a replicating lineage and occupy a particular ecological niche". A "polythetic" class is one whose members have several properties in common, although they do not necessarily all share a single common defining one. Members of a virus species are defined collectively by a consensus group of properties. Virus species thus differ from the higher viral taxa, which are "universal" classes and as such are defined by properties that are necessary for membership.
- ^ Delwart EL. Viral metagenomics. Rev. Med. Virol.. 2007;17(2):115–31. doi:10.1002/rmv.532. PMID 17295196.
- ^ King AMQ, Lefkowitz E, Adams MJ, Carstens EB. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier; 2011. ISBN 0-12-384684-6. p. 6.
- ^ Virus Taxonomy 2009. International Committee on Taxonomy of Viruses. Retrieved on December 24, 2011.
- ^ ICTV Master Species List 2009
- This Excel file contains the official ICTV Master Species list for 2009 and lists all approved virus taxa. This is version 10 of the MSL published on August 24, 2011. Retrieved on December 24, 2011
- ^ Temin, H. M.; Baltimore, D. (1972). "RNA-directed DNA synthesis and RNA tumor viruses". Advances in virus research. Advances in Virus Research. 17: 129–186. doi:10.1016/S0065-3527(08)60749-6. ISBN 9780120398171. PMID 4348509.
- ^ Baltimore D. The strategy of RNA viruses. Harvey Lect.. 1974;70 Series:57–74. PMID 4377923.
- ^ van Regenmortel MH, Mahy BW. Emerging issues in virus taxonomy. Emerging Infect. Dis.. 2004;10(1):8–13. PMID 15078590.
- ^ Mayo MA. Developments in plant virus taxonomy since the publication of the 6th ICTV Report. International Committee on Taxonomy of Viruses. Arch. Virol.. 1999;144(8):1659–66. doi:10.1007/s007050050620. PMID 10486120.
- ^ de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324(1):17–27. doi:10.1016/j.virol.2004.03.033. PMID 15183049.
