Wikipedia:Reference desk/Science: Difference between revisions
SteveBaker (talk | contribs) →Speed: Lorentz factor...Bugs - please stop guessing. |
|||
| Line 458: | Line 458: | ||
:I don't know who posted that image from the Nutt et al paper, but it should be noted that the diagram is slightly flawed since the addictivity of cocaine doesn't take [[crack cocaine]] into consideration, which is ''possibly'' more psychologically addictive than heroin. Also, the methodology of that study isn't as scientific as one would assume for a Lancet paper. --[[User:Mark PEA|Mark PEA]] ([[User talk:Mark PEA|talk]]) 20:16, 22 January 2010 (UTC) |
:I don't know who posted that image from the Nutt et al paper, but it should be noted that the diagram is slightly flawed since the addictivity of cocaine doesn't take [[crack cocaine]] into consideration, which is ''possibly'' more psychologically addictive than heroin. Also, the methodology of that study isn't as scientific as one would assume for a Lancet paper. --[[User:Mark PEA|Mark PEA]] ([[User talk:Mark PEA|talk]]) 20:16, 22 January 2010 (UTC) |
||
::I have seen a bbc documentary created from the results of that study, and they claimed a difference in effect between crack and powder cocaine could not be established. |
|||
::I grabbed it from our [[Heroin]] article and pasted it there — insert obligatory hectoring to [[WP:BOLD|be bold]] and go fix it or challenge its inclusion in that article, etc. [[User:Comet Tuttle|Comet Tuttle]] ([[User talk:Comet Tuttle|talk]]) 21:20, 22 January 2010 (UTC) |
::I grabbed it from our [[Heroin]] article and pasted it there — insert obligatory hectoring to [[WP:BOLD|be bold]] and go fix it or challenge its inclusion in that article, etc. [[User:Comet Tuttle|Comet Tuttle]] ([[User talk:Comet Tuttle|talk]]) 21:20, 22 January 2010 (UTC) |
||
Revision as of 03:14, 23 January 2010
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
January 19
Circuit Problem
Imagine some resistors set in parallel in a circuit (there may be more elements to the circuit). Kirchoff's law says that, for any path the current might take, it's change in potential must be the same. Why is this true (ie what forces the electrons all to lose the same amount of potential (or energy or wtv))? —Preceding unsigned comment added by 173.179.59.66 (talk) 00:26, 19 January 2010 (UTC)
- I'm not sure it is correct to attribute this to a "force". An electron travels through the path of least resistance, so as it loses potential it increases the resistance of the path it took in the conductor, mainly due to heat. This means the next electron behind it won't take exactly the same path, it will take the next best path. This all happens at the speed of light, so when a current is actually flowing through all the parallel resistors it only makes sense that all the electrons lose the same potential. When any one electron has more or less potential then the others around it, it takes a slightly more or less (respectively) "difficult" path in the circuit and then loses more or less potential until it is equal. Otherwise all the current would ravel through the path of absolute least resistance, regardless of how many resistors are in parallel. Sorry my explanation is a little obtuse, maybe someone has a more elagant answer.Vespine (talk) 01:10, 19 January 2010 (UTC)
- This sounds a bit like a homework question, so think about what would happen otherwise. If some lost less potential, you'd have electrons gaining potential as they travel through the loop. A 5V battery can't give a potential higher than that, it would be a violation of the conservation of energy. ~ Amory (u • t • c) 01:16, 19 January 2010 (UTC)
Empirical studies of emotion
Is there any scientific or experimental backing to confirm that the words we use to describe emotion are valid and actually reflect objective reality? For example we have common words that describe colours, and these are underpinned by scientific studies of colour in terms of the spectrum and three colour-detecting cells in the human retina. Have any underlying dimensions been experimentally discovered for emotions? Or are we just stuck at the qualitative level? 89.240.50.241 (talk) 00:52, 19 January 2010 (UTC)
- Have you checked our Emotions article? Mitch Ames (talk) 00:55, 19 January 2010 (UTC)
- You can't quantify emotion any more than you can explain how chocolate tastes. There is a thing called internal validity that measures how a term is used by different people at different times. That is to ensure that what you're calling 'anger' isn't a scattershot representation of all sorts of different negative moods. That sort of thing. Vranak (talk) 05:50, 19 January 2010 (UTC)
- Well we can quantify emotions. I think it is commonly accepted that fear is associated with the fight-or-flight response, which is activated by the sympathetic nervous system; thus we could do a controlled study where adrenaline and its metabolites are measured from samples of blood taken, place it on a graph where subjects report a specific emotion. Look at Graph B on this picture to see what I mean (this is a measure of amphetamine though, not adrenaline).
- If you mean that we can never "know" what a person is feeling, then we are talking about something else known as the problem of other minds. This doesn't mean we can't quantify things, as the OP already mentioned that the colour spectrum is always the same, although we can't "know" what other people are actually seeing (or whether they aren't a philosophical zombie). (Sorry for the scruffy structure of this post, in a hurry) --Mark PEA (talk) 10:46, 19 January 2010 (UTC)
- The science on this goes all the way back to Darwin's book The Expression of the Emotions in Man and Animals. Also very influential is Paul Ekman's work showing that cultures all across the world use very similar facial expressions for basic emotions. Looie496 (talk) 18:08, 19 January 2010 (UTC)
So the answers are No, Yes. 78.151.106.238 (talk) 19:13, 19 January 2010 (UTC)
- Huh? The answers are yes there is experimental backing (going back to Darwin) that the words we use reflect experimental reality, yes underlying dimensions have been discovered (by Ekman among others), no we aren't just stuck at the qualitative level. For a guide to research on the underlying neural bases of emotion, the books and articles by Joseph LeDoux are a good place to start. Regards, Looie496 (talk) 19:34, 19 January 2010 (UTC)
From what I've read in the Ekman article, it seems that he has only shown that his categories have Social reality, not the underlying physiological mechanisms as with colour and cells in the retina. Money now or belief in witchcraft in the past had similar universal social reality but with no underlying physiological basis. So the answers are still No and Yes, with the possible exception of fear. Believing social reality to be objective truth is what the far right do. 92.29.57.199 (talk) 11:19, 20 January 2010 (UTC)
- Well, he showed more than that. Social reality would mean, for example, that all members of a given community understand a smile in the same way. What Ekman showed is that isolated cultures from all across the world understand a smile in the same way. That takes us from social reality to biological reality, as I understand it. Certainly Ekman thought that that was what he had done. Looie496 (talk) 17:40, 20 January 2010 (UTC)
People from different cultures believe in witchcraft or money, and neither have an underlying physiological mechanism. As far as I am aware Ekman never demonstrated a physiological mechanism for emotion, as with colour and retinal cells. 92.29.57.199 (talk) 21:34, 20 January 2010 (UTC)
illusion of wheel's direction
how do we get an illusion of a wheel of a car moving in backward direction,when car is at great speed. —Preceding unsigned comment added by Myownid420 (talk • contribs) 02:28, 19 January 2010 (UTC)
- See Wagon-wheel effect. Nanonic (talk) 02:37, 19 January 2010 (UTC)
- Damn, too slow! 218.25.32.210 (talk) 02:37, 19 January 2010 (UTC)
- There is an absolutely brilliant depiction of the Wagon-wheel/stroboscopic effect on YouTube.HERE The film/video camera shutter is synchronised with the Helicopter rotors rotation. As a result the main rotor appears stationary as the helicopter flies around. --220.101.28.25 (talk) 10:00, 19 January 2010 (UTC)
- In respect to
 , it's not that the effect occurs, but rather than the picture actually does reverse -- if we want to demonstrate the illusion, why does it have to make it happen artificially? (For example, This spinning girl illusion works without "cheating.") DRosenbach (Talk | Contribs) 13:18, 19 January 2010 (UTC)
, it's not that the effect occurs, but rather than the picture actually does reverse -- if we want to demonstrate the illusion, why does it have to make it happen artificially? (For example, This spinning girl illusion works without "cheating.") DRosenbach (Talk | Contribs) 13:18, 19 January 2010 (UTC)
- Uh, that's a completely different effect. 17:35, 19 January 2010 (UTC)
- In respect to
- There is an absolutely brilliant depiction of the Wagon-wheel/stroboscopic effect on YouTube.HERE The film/video camera shutter is synchronised with the Helicopter rotors rotation. As a result the main rotor appears stationary as the helicopter flies around. --220.101.28.25 (talk) 10:00, 19 January 2010 (UTC)
- Damn, too slow! 218.25.32.210 (talk) 02:37, 19 January 2010 (UTC)
- That image isn't cheating: the whole idea is that the two directions of motion are aliased with the frequency of update in the recording (be it a film or a GIF) and thus are indistinguishable. Given only the final product and no contextual clues, it's impossible to say whether it "actually" reverses or not. --Tardis (talk) 18:46, 19 January 2010 (UTC)
- Yep - DRosenbach is not understanding the problem. The spinning girl illusion is due to a lack of depth information - the silhouette of a clockwise rotating dancer looks identical to that of an anticlockwise rotation - so your brain can't understand which it is and flips back and forth between representations. The animated GIF is true temporal aliassing - which is a completely different illusion. SteveBaker (talk) 20:14, 19 January 2010 (UTC)
- That image isn't cheating: the whole idea is that the two directions of motion are aliased with the frequency of update in the recording (be it a film or a GIF) and thus are indistinguishable. Given only the final product and no contextual clues, it's impossible to say whether it "actually" reverses or not. --Tardis (talk) 18:46, 19 January 2010 (UTC)
- In computer graphics, we call this "temporal aliassing".
- The easiest way to think about this is to imagine a wheel with three identical, equally spaced, spokes. In a TV or movie image or computer graphics or something, if the wheel rotates clockwise exactly 120 degrees from one still image to the next then every picture of the wheel would appear to be identical - even though a different one of the spokes would be at the top of the picture each time. Hence, it would not appear to rotate even though it's spinning really fast. However, if the wheel were to rotate only 119 degrees each time then there would be a visual conundrum: Did the wheel rotate 119 degrees clockwise - or one degree anticlockwise? In either case, the resulting series of images would be just the same. Our eyes & brains seem to prefer the slower rotation - so a wheel that's spinning at 119 degrees per frame seems to be rotating slowly backwards. As the wheel slows down, this effect persists - so if it rotates 100 degrees clockwise, our brains will insist that it's rotating 20 degrees anticlockwise...all the way down to 60 degrees. At 60 degrees per frame, we would get the same image whether we rotated 60 degrees forwards or 60 degrees backwards...the image would be identical. Now our poor brains can't figure out what's going on and instead of seeing rotation, we see a kind of flickery 6 spoked wheel! Once you get below 60 degrees per frame, we again have two interpretations - 59 degrees clockwise or 61 degrees anticlockwise. Again, our brains prefer the lower number - so FINALLY, we see what's really going on - a wheel rotating clockwise at 59 degrees per frame.
- The angle below which everything looks normal is therefore exactly half of the spacing of the spokes. If you have a 4 spoked wheel, the anomaly happens when it's spinning at 45 degrees per frame or more...for a 36 spoked wheel, it's only got to be rotating at 5 degrees per frame to look bad. That's why the effect is called the "Wagon wheel effect" - because in the days of early cinema, we had maybe only 24 frames per second - and the stage coach in the cowboy movies that were popular back then only had to rotate fairly slowly to provoke the problem. Wagon wheels have a lot more spokes than most other kinds of wheel! It is probably also the case that filming out in the bright sunny desert where most cowboy movies were made means that the shutter time on the camera had to be kept short - that reduces the effect of motion-blur which greatly enhances the effect. In computer graphics, it takes a lot of effort to simulate motion blur and (in effect) we have an infinitely short "shutter time" - that means that this effect, which had gone largely unnoticed through the era of cars and modern TV cameras is now beginning to show up again.
- What's kinda mind-blowing is that if you merely paint one of the spokes a different color - the illusion more or less vanishes. By breaking the rotational symmetry, the wheel now has to rotate 180 degrees per frame in order to cause this effect - and that's a really fast-moving wheel. We use this technique in computer graphics to try to break the illusion - making all of our wheels asymmetric whenever we can (painting the word "GOODYEAR" in white on the sides of tyres is a good example of that).
- SteveBaker that may be the best RefDesk response ever. Cuddlyable3 (talk) 20:56, 19 January 2010 (UTC)
- Nah - I forgot to mention the Nyquist limit and the 'half the sampling frequency' thing - which ties in nicely with half of the angle of rotational symmetry. SteveBaker (talk) 02:55, 20 January 2010 (UTC)
- SteveBaker that may be the best RefDesk response ever. Cuddlyable3 (talk) 20:56, 19 January 2010 (UTC)
- OK...I thought it was an issue of, "if you keep looking at this, it will switch," and that's why I compared it to the spinning/oscillating girl. With my understanding of it, and that's an apparently incorrect understanding as you explain it, my rationale, which is now false, was as follows: If it's only that after a few seconds of watching the little GIF file above that it appears to switch, why does it appear to go in the opposite direction even if one doesn't look at it for the first half of the video. DRosenbach (Talk | Contribs) 02:42, 20 January 2010 (UTC)
- Nope - this is something completely different. If my three-spoked wheel example has the wheel rotating at 110 degrees per frame, the backwards 10 degrees per frame that you think you're seeing is a rock-solid effect, you can't keep looking at it and see it 'switch'. The GIF is changing direction because the animation is slowly increasing in speed until it hits the temporal aliasing speed - then, although the speed is still increasing, it appears to slow down, stop and then reverse. You can visualize that in the same way we did with the three-spoked wheel. Imagine a video shot out of the window of a car that's driving along parallel to a really long picket fence with vertical strips every 12 inches. If you drive at 12 inches per frame, the fence seems stationary - if you drive at 11 inches per frame, it seems to be moving backwards - at 6 inches per frame, you see twice the number of fence posts - but kinda flickery - at below 6 inches per frame, everything looks normal. In the GIF animation, the green waves are like the fence panels. SteveBaker (talk) 02:55, 20 January 2010 (UTC)
- Thanx! (even though this wasn't my question :) DRosenbach (Talk | Contribs) 04:10, 21 January 2010 (UTC)
- This is more than just the "wagon-wheel effect" from old westerns. I saw an ultra-modern car TV ad the other day, and the same thing was going on. One would think with digitization they could make it "look right". But maybe people are so used to seeing it, that if it looked "right" they would think it looked phony? ←Baseball Bugs What's up, Doc? carrots→ 04:15, 21 January 2010 (UTC)
- Thanx! (even though this wasn't my question :) DRosenbach (Talk | Contribs) 04:10, 21 January 2010 (UTC)
- Nope - this is something completely different. If my three-spoked wheel example has the wheel rotating at 110 degrees per frame, the backwards 10 degrees per frame that you think you're seeing is a rock-solid effect, you can't keep looking at it and see it 'switch'. The GIF is changing direction because the animation is slowly increasing in speed until it hits the temporal aliasing speed - then, although the speed is still increasing, it appears to slow down, stop and then reverse. You can visualize that in the same way we did with the three-spoked wheel. Imagine a video shot out of the window of a car that's driving along parallel to a really long picket fence with vertical strips every 12 inches. If you drive at 12 inches per frame, the fence seems stationary - if you drive at 11 inches per frame, it seems to be moving backwards - at 6 inches per frame, you see twice the number of fence posts - but kinda flickery - at below 6 inches per frame, everything looks normal. In the GIF animation, the green waves are like the fence panels. SteveBaker (talk) 02:55, 20 January 2010 (UTC)
- OK...I thought it was an issue of, "if you keep looking at this, it will switch," and that's why I compared it to the spinning/oscillating girl. With my understanding of it, and that's an apparently incorrect understanding as you explain it, my rationale, which is now false, was as follows: If it's only that after a few seconds of watching the little GIF file above that it appears to switch, why does it appear to go in the opposite direction even if one doesn't look at it for the first half of the video. DRosenbach (Talk | Contribs) 02:42, 20 January 2010 (UTC)
circuits
what will happen if we put a resistor of,say, R1 and a simple wire say copper wire in parallel in a circuit made up of copper wire,with a battery and a switch only. what will be the resistance in the circuit.could it be solved like that
1/0 + 1/R1 = 1/Rp (as the resistance of copper wire is 0)
where Rp is total resistance in parallel. —Preceding unsigned comment added by Myownid420 (talk • contribs) 02:51, 19 January 2010 (UTC)
- The resistance of a copper wire is not zero, see here for some numbers. From my knowledge of electronics, what you seem to be suggesting would most likely result in a short-circuit. Such a circuit would have close to (but not exactly) zero so you can pretty much ignore the resistor since almost all the current is going to flow through the copper wire. Also, you probably shouldn't try to divide by zero. - Akamad (talk) 03:06, 19 January 2010 (UTC)
- The current will be large and limited by the resistance in the battery. As Akamad said you can ignore the parallel resister. If there is no resistance in the wire or battery the current will still be limited by inductance, and will grow linearly with timeGraeme Bartlett (talk) 08:12, 19 January 2010 (UTC)
plate changing speeds
Is this possible the plate movement will start out slow then end up moving fast? Because Africa 100 million years ago move faster now it slow to 2 cm/year. Is this possible Antarctica could eventually move as fast as Australian plate? WHat changes the speed motion? Could Australia eventually slow down?--69.228.145.57 (talk) 04:49, 19 January 2010 (UTC)
- It is possible, and the direction of movement could change over time. What happened in the past can be found by the geomagnetic reversal timing signatures on the ocean floor. Predicting the future is more difficult. One book I read stated that the circulation in the mantle is turbulent and so it varies over time and is not simply predictable. Graeme Bartlett (talk) 08:42, 19 January 2010 (UTC)
nuary 2010 (UTC)
Tree of Life in circular form
A question rather than an answer from me for a change. The current Evolution articles' template features a diagram of the "Tree of life" in a space-saving circular layout, and in recent years I've seen other similarly circular versions elsewhere. Does anyone know where this general circular layout of it originated? (The reason I ask is that, while desk-editing a school science textbook in 1990, I was asked by the authors to design and/or source a number of diagrams and other pictures, including a very simple Tree of life, and came up with just such a near-circular layout in order to save space on the page. Although I had no previous example consciously in mind it seems very unlikely that circular Trees of Life weren't already in use and quite probable that I'd seen one before.) 87.81.230.195 (talk) 04:59, 19 January 2010 (UTC)
- It has developed gradually. At [1] you can see Darwin's own sketch where the branches from the roots go in different directions. This hand-made tree: [2] from Science in 1997 uses a similar space-saving layout. The circle appears naturally as you add more and more branches. The web-based generator for the image you refer to can be found at [3]. The company behind it claims it's a novel type of visualization, so according to them the style orignated in 2006. Though in my opinion, the main driver is large tree-of-life databases. EverGreg (talk) 09:45, 19 January 2010 (UTC)
- Some more examples: Carl Woese in 1988 (figure 4) [4] Cover of Molecular systematics 1996: [5]. There's also an example you may have come across in school. There's a circular diagram where each kid in class starts off in the centre and then moves outward in a circular tree according to genetic properties such as gender, ability to roll your tongue e.t.c. It's not a phylogenetic tree, but gives an image of genetic similarity. EverGreg (talk) 10:06, 19 January 2010 (UTC)
- Thanks for those suggestions, EverGreg, but some of those examples are, like Darwin's familiar original (published, incidentally, by the same firm I was working for), radial rather than circular, while the circular ones postdate my own usage. I certainly never encountered your school exercise (which would have to date back nigh-on four decades for me to have done so). Surely someone can come up with a good pre-1990 example to save me from the hubris of suspecting I may have originated it myself? 87.81.230.195 (talk) 10:56, 19 January 2010 (UTC)
Electrical Heating Distribution
So the above thread about heat pumps has me wondering about electrical heating in general. In what parts of the world is electrical heating commonly used to heat buildings? Here in (Suburban) Minnesota gas heating is the norm, and electrical heating is pretty much unheard of, except for maybe a small space heater in an ice shack or something. I understand that in the UK it's pretty common to take advantage of lower energy prices at night with a storage heater. We also had someone from Australia and Texas say that they had electrical heating. It seems to be that electrical heating is more common more temperate places. Do southern Europeans use electrical heating on a large scale? What about northern Europeans? I assume that Russians use natural gas (because they have enough of it), but I don't really know. What about in developed Asian countries like Japan? Buddy431 (talk) 05:23, 19 January 2010 (UTC)
- Electric heating is cheap to install, so low cost systems in Australia use a simple fan over hot element heating. More sophisticated systems could be in slab or floor eating or the storage system using off peak electricity you mentioned. Reverse cycle systems can be quite efficient giving a gain of 4x the energy consumed. To use gas, reticulation infrastructure is needed in the form of plumbing or big gas bottles. Some do use this. Coal in furnaces is rare, and in the past there used to be oil heaters. Before this burning wood in fireplaces or stoves was common. Graeme Bartlett (talk) 08:09, 19 January 2010 (UTC)
- I had electric baseboard heaters when I lived in Tennessee. And I wasn't the only one. I knew several people, especially those who were living in trailers who had electric heat. Dismas|(talk) 09:51, 19 January 2010 (UTC)
- In some parts of canada hydroelectric electricity is cheaper than gas. (Probably due to subsidies.) So electric heat is used. Otherwise electric heat is used when the installation cost of a gas burner is more than the savings of using gas vs electricity. Electric heat is very cheap to install. U.S. view: This is typically in the south where it doesn't get that cold. You have sort of a continuum - in the north, all gas. No A/C, so it's usually radiators (water). In the middle it's electric heat, and no A/C at all. In the south you have A/C (meaning the ductwork is anyway already there), so they use a central heat source, and they do gas. This is generalizing a lot of course. Also some places do not have gas service, and oil in not available. So electric is the default. These days, even in the north new construction comes with A/C, and the price of electricity has gone up more than gas, so it's pretty much all gas if it's available. Ariel. (talk) 10:27, 19 January 2010 (UTC)
- Electrical heating is clearly the norm in Norway, a developed country in the north. Historically, we had very cheap hydroelectricity, you certainly don't need subsidies for this. Prices do rise as transmission capacity to the rest of Europe improves, though. There are (almost?) no distribution nets for gas in Norway, and I don't think it would be economic to build those; gas is piped from Norway's large sub-ocean gas fields via stations on the coast across the North Sea to Britain and the Netherlands. Large buildings do use oil for fuel though. Many single-unit houses have wood ovens as a supplement. I think electricity is the norm in Sweden and Finland as well. Jørgen (talk) 12:55, 19 January 2010 (UTC)
- Electricity prices for the Nordic countries are here, in EUR/MWh, it would be interesting if some US users could compare this to their local heating prices (assuming 100% electricity efficiency). The electricity market in Norway is very competitive, I think the end-user prices are very close to the "market trading" prices (though transmission cost of 0,39 NOK/kWh (ca 7 US cents) comes in additition (at least that's the price in my area)) Jørgen (talk) 13:11, 19 January 2010 (UTC)
- Here is a relatively informative article per your question, as you can see from the graph $.07 US/kwh is pretty darn low, a price almost no one in the US has seen since 2005. Depending on the natural gas spot market in the US, the price tends to be around 1/3 as much to get a BTU from NG compared to electricity (assuming the NG furnace is of modern efficiency). --Jmeden2000 (talk) 16:52, 19 January 2010 (UTC)
- Electricity prices for the Nordic countries are here, in EUR/MWh, it would be interesting if some US users could compare this to their local heating prices (assuming 100% electricity efficiency). The electricity market in Norway is very competitive, I think the end-user prices are very close to the "market trading" prices (though transmission cost of 0,39 NOK/kWh (ca 7 US cents) comes in additition (at least that's the price in my area)) Jørgen (talk) 13:11, 19 January 2010 (UTC)
- In some parts of Russia and Ukraine, they still use coal gas... 24.23.197.43 (talk) 07:22, 23 January 2010 (UTC)
difference between sea and ocean
what is the technical differnce in the definition of a sea and an ocean?Denito (talk) 07:46, 19 January 2010 (UTC)
- Sea is small and Ocean is big. eg sea of Galilee is pretty miniature, but famous. The oceans currently on earth are pretty fixed in number, but perhaps your definition request is important to naming oceans in the geological past of earth, or on other planets. The Sea of Marmara is claimed to be the smallest sea, but sea of Galilee is smaller. According to Wikipedia a Sea is large, and an ocean is major. Graeme Bartlett (talk) 08:02, 19 January 2010 (UTC)
- Even worse though, while sea of Galilee is a traditional name, it is actually a freshwater lake. Googlemeister (talk) 14:41, 19 January 2010 (UTC)
- To further confuse things, some seas are part of a larger ocean (for example, the Carribean Sea is part of the Atlantic Ocean; the Andaman Sea is part of the Indian Ocean), whereas other seas, such as the Baltic Sea are separate bodies of water that are connected to oceans. See marginal sea and mediterranean sea. Gandalf61 (talk) 10:31, 19 January 2010 (UTC)
- Oceans abut and separate continents. A sea is just some water on which one can sail a boat. Cuddlyable3 (talk) 13:15, 19 January 2010 (UTC)
- Hmmm. The Bering Sea separates North America and Asia; the Red Sea lies between Asia and Africa; and the Mediterranean Sea has coastlines in Europe, Africa and Asia. But they are not oceans. Gandalf61 (talk) 15:10, 19 January 2010 (UTC)
- Indeed. I did not claim that oceans have a monopoly on what they do. Cuddlyable3 (talk) 20:44, 19 January 2010 (UTC)
- Hmmm. The Bering Sea separates North America and Asia; the Red Sea lies between Asia and Africa; and the Mediterranean Sea has coastlines in Europe, Africa and Asia. But they are not oceans. Gandalf61 (talk) 15:10, 19 January 2010 (UTC)
- Oceans abut and separate continents. A sea is just some water on which one can sail a boat. Cuddlyable3 (talk) 13:15, 19 January 2010 (UTC)
- To further confuse things, some seas are part of a larger ocean (for example, the Carribean Sea is part of the Atlantic Ocean; the Andaman Sea is part of the Indian Ocean), whereas other seas, such as the Baltic Sea are separate bodies of water that are connected to oceans. See marginal sea and mediterranean sea. Gandalf61 (talk) 10:31, 19 January 2010 (UTC)
- Because "ocean" is of European origin, it should be obvious that the use came from early Europeans. Add to the concept of an ocean the earlier fact that the world was flat, an ocean was the body of water that, if you sailed through it, led to the edge of the world. The world consisted of Europe, Asia, and Africa. The four oceans (just four at the time) were to the north, east, south, and west of the world. Once the world was proven round, there was no need to change the name. -- kainaw™ 15:24, 19 January 2010 (UTC)
- Not to get too humanities on you, but that brief history of European knowledge of geography is ridiculously inaccurate. The Earth was known to be round in ancient and medieval times: for example, see our article on mappa mundi. 86.178.230.208 (talk) 17:20, 19 January 2010 (UTC)
- I think there is also considerable geographical variation in the use of the term. I've noticed that Americans use the word "Ocean" in many contexts where the British would say "Sea". That may be because the USA is bounded by a couple of oceans where the Brit's have the North Sea on one side and an Ocean on the other. But it's only a matter of linguistics - there isn't any science behind it, beyond some vague concept of size. However, people still talk about "Sailing the Seven Seas"...when they probably mean something like "Four oceans and three seas". SteveBaker (talk) 19:11, 19 January 2010 (UTC)
- To be more "humanities" in my answer... The English term "Ocean" comes from the Greek Oceanus. It is a concept of a Greek god represented as a world ocean upon which the world floats. The "flat" world concept is a simplification of the of concept of a round world floating in Oceanus. Many cultures named the coastal waters, but continued to refer to the unknown waters as being part of Oceanus, or an Ocean. The modern view of the world ocean is slightly romanticized with the Greek concept of an ocean. Atlantic comes from Greek Atlas, an offspring of Oceanus. Arctic comes from Greek Arktikos - the great bear in the northern stars. Antarctic is away from the bear. Pacific was named a long time later with a Latin name. It seems to me that Magellan should have known enough to give it a Greek-based name. However, the concept of an unknown ocean is what is different about the oceans and the seas. In modern times, very little is unknown. We just continue to use the terms here and there out of tradition. -- kainaw™ 20:22, 19 January 2010 (UTC)
- [citation needed] --Mr.98 (talk) 20:37, 19 January 2010 (UTC)
- Did you consider looking at Ocean, Oceanus, World ocean, or Atlas (mythology) before requesting a citation? -- kainaw™ 21:05, 19 January 2010 (UTC)
- Our article Oceanus attributes that view to some scholars. Do you have a good secondary source we could use to improve the article? Our article World ocean doesn't really talk about it, because it is discussing the actual, existent world ocean. Our article Ocean briefly mentions the idea under the heading culture, but has no sources. Our article Atlas of course mentions that he was considered Oceanus's son, but I don't think anyone was disputing that. It says nothing about this (presumably old even to the ancient Greeks) conception of the world. None of these articles mention four oceans to the north, south, east and west of the world. So maybe Mr.98 did read the articles. In any case, some citation would be welcome to improve these articles (as well as our collective answer). 86.178.230.208 (talk) 18:43, 20 January 2010 (UTC)
- I just don't buy the explanation you're giving for how the distinctions came about in our modern world. The original poster is asking about how we might define things today, and appealing to Greek myths is probably incorrect. Geography went through quite a few changes since the Greeks. --Mr.98 (talk) 21:51, 20 January 2010 (UTC)
- Our article Oceanus attributes that view to some scholars. Do you have a good secondary source we could use to improve the article? Our article World ocean doesn't really talk about it, because it is discussing the actual, existent world ocean. Our article Ocean briefly mentions the idea under the heading culture, but has no sources. Our article Atlas of course mentions that he was considered Oceanus's son, but I don't think anyone was disputing that. It says nothing about this (presumably old even to the ancient Greeks) conception of the world. None of these articles mention four oceans to the north, south, east and west of the world. So maybe Mr.98 did read the articles. In any case, some citation would be welcome to improve these articles (as well as our collective answer). 86.178.230.208 (talk) 18:43, 20 January 2010 (UTC)
- Did you consider looking at Ocean, Oceanus, World ocean, or Atlas (mythology) before requesting a citation? -- kainaw™ 21:05, 19 January 2010 (UTC)
- [citation needed] --Mr.98 (talk) 20:37, 19 January 2010 (UTC)
- To be more "humanities" in my answer... The English term "Ocean" comes from the Greek Oceanus. It is a concept of a Greek god represented as a world ocean upon which the world floats. The "flat" world concept is a simplification of the of concept of a round world floating in Oceanus. Many cultures named the coastal waters, but continued to refer to the unknown waters as being part of Oceanus, or an Ocean. The modern view of the world ocean is slightly romanticized with the Greek concept of an ocean. Atlantic comes from Greek Atlas, an offspring of Oceanus. Arctic comes from Greek Arktikos - the great bear in the northern stars. Antarctic is away from the bear. Pacific was named a long time later with a Latin name. It seems to me that Magellan should have known enough to give it a Greek-based name. However, the concept of an unknown ocean is what is different about the oceans and the seas. In modern times, very little is unknown. We just continue to use the terms here and there out of tradition. -- kainaw™ 20:22, 19 January 2010 (UTC)
- I think our definition on the ocean page is pretty good. "An ocean is a major body of saline water, and a principal component of the hydrosphere." Now, obviously the line between what isn't a principle component of the hydrosphere or not is somewhat arbitrary, but the acknowledged oceans dwarf any candidate large seas considerably. The smallest ocean is some five times larger than the largest sea, while the rest of the oceans are some 20X larger. IMO the only questionable inclusion is whether the Arctic Ocean counts as an "ocean" or not. The Arabian and South China seas are both very small by comparison to the other oceans. --Mr.98 (talk) 20:37, 19 January 2010 (UTC)
- Well...kinda...but the division of a more or less contiguous irregular shape into regions is entirely arbitary anyway. Where exactly the South China sea ends and the Pacific starts is a totally arbitary line. SteveBaker (talk) 02:40, 20 January 2010 (UTC)
- Agreed for the most part. (In some cases, underlying geology does make certain waters have a different character than others, I believe, which would seem like a good reason to delineate them, if you were a sailor.) --Mr.98 (talk) 21:51, 20 January 2010 (UTC)
- Well...kinda...but the division of a more or less contiguous irregular shape into regions is entirely arbitary anyway. Where exactly the South China sea ends and the Pacific starts is a totally arbitary line. SteveBaker (talk) 02:40, 20 January 2010 (UTC)
Oceans occur in the unlikeliest places. Thomas Babington Macaulay talked of The old philosopher is still among us ...blinking, puffing, rolling his head, drumming with his fingers, tearing his meat like a tiger, and swallowing his tea in oceans. -- Jack of Oz ... speak! ... 00:29, 20 January 2010 (UTC)
Is one difference that all the oceans on our planet connect together wrapping around the planet while the seas are mostly disconnected from being a solid unit, themselves separated from each other either by oceans or by land? --Neptunerover (talk) 09:08, 20 January 2010 (UTC)
- Hmmmm. Black Sea, Aegean Sea, Mediterranean Sea, Ionian Sea and Adriatic Sea. Cuddlyable3 (talk) 13:56, 20 January 2010 (UTC)
- No. It really doesn't take much effort to find counter-examples for those kinds of propositions (it would be nice if you did that rather than just posting wild speculation). For example:
- The Mediterranean Sea does not connect to any oceans directly because the Alborean Sea is in the way.
- The Alborean Sea does connect to the Atlantic ocean but only via the straights of Gibralta (is that a 'connection' or not?).
- The Aral Sea is completely land-locked and doesn't connect to any other bodies of water whatever.
- The Sargasso Sea is completely surrounded by the Atlantic ocean and touches no land or other regions of water. It's boundaries are defined by ocean currents and as such, it doesn't even stay in the same place from one year to the next!
- The Argentine Sea is nothing much more than an arbitary strip of water that is some unspecified number of miles wide adjoining a vaguely delimited stretch of coastline on the edge of the Atlantic ocean.
- There is no solid rule here - it's a mess. Which is fine if you are just giving random bits of water pretty names - but has zero scientific meaning.
- SteveBaker (talk) 14:06, 20 January 2010 (UTC)
Tsk, tsk. "The ALBORAN Sea does connect to the Atlantic ocean but only via the STRAITS OF GIBRALTER." Cuddlyable3 (talk) 17:02, 20 January 2010 (UTC)
- Where is the "Alboran Sea", anyway? On my map, there's not such a thing, and the Straits of Gibraltar connect the Mediterranean directly to the Atlantic Ocean. Has that region's geography undergone some major tectonic changes? 24.23.197.43 (talk) 07:19, 23 January 2010 (UTC)
- With the exception of oddities like the Sea of Galilee, seas and oceans are all saltwater, right? Misnamed things stay that way. Like Cape Cod, which is not a cape, it's a peninsula. Or, thanks to the canal, it's an island. ←Baseball Bugs What's up, Doc? carrots→ 17:19, 20 January 2010 (UTC)
- When you come right down to it - geographers have a hard time of keeping names and definitions in order. (I write this from somewhere in the middle of the island of northandsouthamerica - a piece of land surrounded entirely by water). SteveBaker (talk) 00:09, 21 January 2010 (UTC)
- And as with Cape Cod, the canal probably creates two islands out of one. Meanwhile, ponder this, and not just the bizarre fact that most of us live "in continents": Sometimes within a continent you'll find a lake that's self-contained. For example, Crater Lake in Oregon. That body of water has an island within it, an extinct volcanic cone. That island has various little pools of water. So that island also has small "lakes" within it. And some of those little pools have little bitty islands within them... and so on. Hey, why do I suddenly feel like this should be on the math page? ←Baseball Bugs What's up, Doc? carrots→ 04:38, 21 January 2010 (UTC)
- When you come right down to it - geographers have a hard time of keeping names and definitions in order. (I write this from somewhere in the middle of the island of northandsouthamerica - a piece of land surrounded entirely by water). SteveBaker (talk) 00:09, 21 January 2010 (UTC)
- Does depth have anything to do with it? Are there any seas as deep as the shallowest ocean's maximum depth? --Neptunerover (talk) 15:50, 21 January 2010 (UTC)
how do i get annoying stuff like picture of the day , poker ect to stop appearing on my updates? —Preceding unsigned comment added by Killspammers (talk • contribs) 14:24, 19 January 2010 (UTC)
- Do you mean on your newsfeed (items that your friends have posted), on your wall (items that applications have posted whilst you're using them) or on your notifications? If you are asking how to hide certain application updates from friends appearing on your newsfeed, you can hover over the item and a 'Hide' button will appear. On clicking this you will get the option to either hide all posts from this user or all posts from this application. You can also do this by clicking 'Edit options' on the footer bar at the bottom of your feed. To stop applications that you use posting to your wall automatically you have to alter the settings for each of these individually. Open an application and hover over 'Settings' on the header bar at the top of the screen, each application has it's own settings page which you would then be able to see and open. Click on this and go to 'Additional permissions' and untick 'Publish recent activity to my Wall'. If you are asking how to stop certain applications appear in your notifications - click on the notification icon in the bottom right of the screen, click 'See more' to go to the notification settings page and untick those applications you don't want to recieve information from on the right hand side. HTH. Nanonic (talk) 15:17, 19 January 2010 (UTC)
no i want to delete the application from my facebook. how do i do it —Preceding unsigned comment added by Killspammers (talk • contribs) 15:57, 19 January 2010 (UTC)
- Well, you can start by having a look at facebook's own help on this]. Nanonic (talk) 16:33, 19 January 2010 (UTC)
cyclisation of tryptophan
From the indole article: "Since the pyrrollic ring is the most reactive portion of indole, nucleophilic substitution of the carbocyclic (benzene) ring can take place only after N-1, C-2, and C-3 are substituted."
Is this really true if intramolecular substitution is taking place? Say the C2 site is protected, and C3 site is already occupied. I think if the amino group is protected (maybe by simple acid) the carboxy group will attach on the benzene ring. Can someone confirm this? If so, I'm going to change the article. John Riemann Soong (talk) 15:07, 19 January 2010 (UTC)
- Intramolecular effects can beat intrinsic reactivity, but that doesn't change the fact of the intrinsic reactivity. The result for any specific reaction is always a balance (and rationalization based on) of all competing effects. DMacks (talk) 18:50, 19 January 2010 (UTC)
- Yes, but that is a preference for substitution, right? I don't think the article should be speaking in absolute terms. John Riemann Soong (talk) 20:22, 19 January 2010 (UTC)
Woman sentenced to 4 years for torching boyfriend's penis
how is it possible she got only 4 years? why didnt they give her 20 to 40 years or even life ? if a man raped her hed get like 10 years but she does something a million times worse and gets only 4 years? http://www.cbc.ca/canada/montreal/story/2007/02/28/qc-andreerene.html
- Your opinion is that it was "a million times worse" than rape. Others may disagree with you. In any case, to go with your analogy, I'm not sure what the standard sentence for rape is in Canada, but I'd doubt it's anything like "20 to 40 years or even life". Does anyone know? --Dweller (talk) 16:08, 19 January 2010 (UTC)
- According to this website, the maximum possible sentences for sexual assault crimes range depending on the exact conviction, from 6 months to life. The judge gets a lot of discretion as to what exact sentence is issued though. It article implies that the sentencing had a lot to do with mitigating circumstances—the defendant's history of abuse, psychological problems, etc.—which are often taken into account for such things. Whether it is a truly just sentence or not, I don't know, and I'm sure informed people would differ. --Mr.98 (talk) 17:56, 19 January 2010 (UTC)
- Personal opinions but I don't give a shit; "mitigating circumstances" of the rapist should have zero influence on their sentence. I don't give a shit if the rapist is temporally mentally insane or whatever the fuck other excuses they boil up - rape is rape and imo rapists should be locked up for life.
- According to this website, the maximum possible sentences for sexual assault crimes range depending on the exact conviction, from 6 months to life. The judge gets a lot of discretion as to what exact sentence is issued though. It article implies that the sentencing had a lot to do with mitigating circumstances—the defendant's history of abuse, psychological problems, etc.—which are often taken into account for such things. Whether it is a truly just sentence or not, I don't know, and I'm sure informed people would differ. --Mr.98 (talk) 17:56, 19 January 2010 (UTC)
- The Wikipedia Science Reference Desk isn't a discussion forum for opinions, debates, and chats about current events. Perhaps you could find a suitable internet forum for this type of question? TenOfAllTrades(talk) 16:10, 19 January 2010 (UTC)
(Also where's the science in this question? I suppose there's Fire involved but still... 194.221.133.226 (talk) 16:17, 19 January 2010 (UTC)
- I did not find any information out there, other than your link, about this 2007 sentencing, but I would guess that, under the Canadian Criminal Code, section 268, she was probably charged with "aggravated assault", for endangering the life of the complainant, which means she can be imprisoned for up to 14 years. By contrast the penalties for rape in Canada (page 4 of the PDF) are maximums of 10 years ("sexual assault"), 14 years ("sexual assault with a weapon, threats to a third party, or causing bodily harm"), or life ("aggravated sexual assault") — the latter meaning that the rapist also "wounds, maims, disfigures, or endangers the life of the complainant". Those are the maximums — I didn't find any information about why in this specific case she got a lesser prison term (assuming I was even right about the charge brought). I learned two things looking this up — the word "rape" is no longer in the Canadian criminal code; and the first rape law in the USA reduced the penalty significantly if the female victim were single (!). Comet Tuttle (talk) 17:56, 19 January 2010 (UTC)
so why wasent she givin the maximum sentence 14 years? also if you would rather get your genitals set on fire than get raped u need your head examined. —Preceding unsigned comment added by 67.246.254.35 (talk) 18:44, 19 January 2010 (UTC)
- Because a judge, probably in light of the aforementioned mitigating circumstances, decided to sentence her otherwise. --Mr.98 (talk) 19:01, 19 January 2010 (UTC)
- The general idea is that you want to punish unjustified, capricious malice. It was the opinion of the sentencing parties that Ms. Flame didn't have that much malice. Probably they thought she was somewhat justified. She evidently thought she was justified at the time of doing it, and that would be good enough for me if I were a juror. Vranak (talk) 20:30, 19 January 2010 (UTC)
- At least in Germany, and I guess in many other countries, first offenders usually get sentences much below maximum. One reason is that the public prosecutors and judges are civil servants with a life career, i.e. they are professionals and do not have to play to public sentiment. --Stephan Schulz (talk) 22:15, 19 January 2010 (UTC)
- I have tried to find the case on the court's website, but it doesn't seem to be there. (If anyone else wants to try, this seems to be the right site, but searching for René under Court of Quebec Criminal division doesn't find anything - the website is in French, however, which is a language I don't actually speak, so a French speaker may have better luck.) --Tango (talk) 01:50, 20 January 2010 (UTC)
Nothing is worse than rape. /thread —Preceding unsigned comment added by 82.43.91.83 (talk) 22:05, 19 January 2010 (UTC)
- Murder? Mass murder? Torture? Child abuse? Genocide? You are entitled to your opinion, but it is far from a universally held one. --Tango (talk) 01:37, 20 January 2010 (UTC)
- I would rather all those things happen than rape, I would kill myself before rape. Yes, it's not a universally held opinion as you pointed out.
- Again, this is not a debate forum. Please stop it. --Anonymous, 01:50 UTC, January 20/10.
- I initially read the question as "touching" and the punishment seemed, well, excessive. Edison (talk) 18:02, 20 January 2010 (UTC)
- I wonder what the punishment would have been if she coldcocked him? ←Baseball Bugs What's up, Doc? carrots→ 18:38, 20 January 2010 (UTC)
Autocrine signalling
From what I understand, autocrine signalling is where a cell releases a transmitter to purely act on its own receptors, so that it can talk to itself. Is there any good reason for this, or is my premise wrong (i.e. a cell never wants to talk just to itself)? At first glance it appears to be inefficient, probably analogous to sending yourself a text message when you could just store it in the draft folder and avoid the SMS cost. Or writing down "pour yourself a glass of water" and reading it, instead of just pouring yourself a glass of water. --Mark PEA (talk) 17:30, 19 January 2010 (UTC)
- I suppose you've never written a todo list? My first thought about its utility is that it lets the cell react over an extended period of time (the lifetime of the transmitter) to even a transitory stimulus. --Tardis (talk) 18:39, 19 January 2010 (UTC)
- Well the reason humans write todo lists is because their memories can fail them, otherwise they would just "remember" their todo list (it's the same reason why I think in my head, as opposed to speaking all my thoughts, because that would be a waste of energy*). Also I'm not denying that it has utility, it just seems there would be a more effective way of doing this rather than synthesizing large numbers of receptors and transmitters, releasing them to the outside, and then detecting their signal again. Of course this is useful in paracrine signalling or synaptic signalling, where autoreceptors can be used to maintain negative feedback systems. But for 100% autocrine signalling it is a waste of energy.
- *Of course there are reasons to talk to oneself, not for self-communication but to assess one's voicebox, or just being an irrational, drunk idiot :) --Mark PEA (talk) 21:10, 19 January 2010 (UTC)
- There are many biological processes in which amplification of a signal must occur: autocrine signalling is one mechanism for this. Of course it is "inefficient" from an engineering standpoint--- one more example of the difference betweened designed and evolved complex entities. alteripse (talk) 19:14, 19 January 2010 (UTC)
- (ec) I might speculate that this is an example of evolution deciding to 'reuse its code', as a programmer might say. Rather than having to evolve a whole new set of signalling proteins to trigger some sort of signalling cascade in the middle of an existing pathway, autocrine signalling means that a cell can just use already-existing genes and proteins to trigger a signal in the 'usual' way from an 'external' stimulus. While it might not appear to be the most efficient method, it works. (Consider this — let's say that you are standing in front of a photocopier, and you just happen to need a sheet of blank paper. One solution is to try to figure out how to unlatch and open the paper trays, locate the pages of the correct size, remove a single sheet, make sure everything is closed back up properly, and declare success. Another solution is to just push the copy button on the front panel without putting an original on the glass. Presto — out comes the sheet you wanted, no diassembly or fiddling required. Evolution has a habit of reusing old methods with minor tweaks; it's 'easier' than generating whole new pathways from scratch.)
- I'll also note that the definition of autocrine signalling is often a bit broader than simply a single cell acting on itself. It can also include cases where a particular cell signals not just to itself, but also to other cells of the same type. The applications of that sort of signalling are more obvious. Among other things, it allows groups of cells to 'assess' their surroundings by 'polling' their neighbors. A cell can 'sense' whether (for example) a lot of its neighbors are stressed out and respond accordingly. TenOfAllTrades(talk) 19:23, 19 January 2010 (UTC)
- Yes, I can understand it in this sense, although my understanding is that this is a form of paracrine signalling. All the answers still seem to be evidence of Unintelligent Design, as I think with the massive amount of junk DNA, the cell could easily have some genes which allow it to talk to itself intra-cellularly that use less energy than this current way. Of course all of this relies on the premise that autocrine signalling is used purely for self-talking, which it probably doesn't (thus, I'd rather it were called pseudoautocrine signalling, since some paracrine signalling is thrown in). --Mark PEA (talk) 21:10, 19 January 2010 (UTC)
- In general, autocrine signalling refers to messages between cells of the same type (including messages sent and received by the same cell), whereas paracrine signalling involves signalling between cells of different types or lineages. TenOfAllTrades(talk) 21:57, 19 January 2010 (UTC)
- Thanks, my original premise was wrong then. (Retrospectively I now notice that the autocrine article actually states that this type of signalling is amongst the same type of cells). --Mark PEA (talk) 23:48, 19 January 2010 (UTC)
- While autocrine signaling may involve more than one cell, there is a decent argument for a cell signaling itself in this way. For example, T cells use IL-2 in an autocrine loop, upregulating their high-affinity IL-2 receptor when stimulated. The extracellular loop provides a mechanism for nearby cells to sense and modulate the T cell's activation. Similar thing can happen with type I interferon signaling - if RIG-I is activated by dsRNA, interferon beta is one product, which may act in both autocrine and paracrine fashion to promote antiviral responses in both the original cell and its neighbors. I don't think your original premise was wrong at all, if I understood it correctly. -- Scray (talk) 01:48, 20 January 2010 (UTC)
- In your scenario... (1) stimulus tells cell to to signal to itself → (2) cell produces & releases transmitters → (3) transmitters bind to membrane receptors → (4) receptors activate signalling pathways, which tell the cell to synthesize more receptors (upregulation).
- My original question was asking why not just have a scenario where... (1) stimulus tells cell to "signal to itself" → (2) cell activates signalling pathways directly (that receptors in the step 4 above activate), which tell cell to synthesize more receptors. Of course, the answer seems to be that the autocrine signalling also signals to other cells (which opposes my original premise), or that evolution just hasn't had the selection pressure to create new code to do it a slightly more efficient way. --Mark PEA (talk) 10:32, 20 January 2010 (UTC)
- Not only that, Mark, but in a case in which a type of cells is already set-up to receive an external message via SIGNAL A, it would be pretty efficient for that cell type to simply release signal A to set off the desired chain of events, rather than develop an entirely new intracellular pathway (and this rationale would suffice even if your autocrine example is a single cell, such as a lonesome leukocyte, etc. DRosenbach (Talk | Contribs) 04:20, 21 January 2010 (UTC)
- While autocrine signaling may involve more than one cell, there is a decent argument for a cell signaling itself in this way. For example, T cells use IL-2 in an autocrine loop, upregulating their high-affinity IL-2 receptor when stimulated. The extracellular loop provides a mechanism for nearby cells to sense and modulate the T cell's activation. Similar thing can happen with type I interferon signaling - if RIG-I is activated by dsRNA, interferon beta is one product, which may act in both autocrine and paracrine fashion to promote antiviral responses in both the original cell and its neighbors. I don't think your original premise was wrong at all, if I understood it correctly. -- Scray (talk) 01:48, 20 January 2010 (UTC)
- Thanks, my original premise was wrong then. (Retrospectively I now notice that the autocrine article actually states that this type of signalling is amongst the same type of cells). --Mark PEA (talk) 23:48, 19 January 2010 (UTC)
- In general, autocrine signalling refers to messages between cells of the same type (including messages sent and received by the same cell), whereas paracrine signalling involves signalling between cells of different types or lineages. TenOfAllTrades(talk) 21:57, 19 January 2010 (UTC)
- Yes, I can understand it in this sense, although my understanding is that this is a form of paracrine signalling. All the answers still seem to be evidence of Unintelligent Design, as I think with the massive amount of junk DNA, the cell could easily have some genes which allow it to talk to itself intra-cellularly that use less energy than this current way. Of course all of this relies on the premise that autocrine signalling is used purely for self-talking, which it probably doesn't (thus, I'd rather it were called pseudoautocrine signalling, since some paracrine signalling is thrown in). --Mark PEA (talk) 21:10, 19 January 2010 (UTC)
Technical vs managerial career track
I'm hoping you RefDesk folks can refer me to some essay, website, book, etc that discusses the choice faced by a scientist or other technical worker when presented with the opportunity to move into management. (I'd love to discuss this with someone, but I understand the RefDesk isn't the place for it). ike9898 (talk) 17:41, 19 January 2010 (UTC)
- See Dilbert (sorry). Looie496 (talk) 18:00, 19 January 2010 (UTC)
- I'm not familiar with any books confronting this career choice, but googling manager career site:slashdot.org will yield many threads on the subject. Warning: Many "contributors" are grousing computer programmers who believe all management is counterproductive micromanagement. If you're looking for books on technical management (rather than focusing on the career choice which was your actual question), The Mythical Man-Month is considered a classic of software development management. Comet Tuttle (talk) 18:33, 19 January 2010 (UTC)
- Managers get paid more and they are the boss. 78.151.106.238 (talk) 19:08, 19 January 2010 (UTC)
- In my particular situation (US government), the difference in pay is small and and in my current situation as a researcher, I have a fair degree of autonomy. Both these factors reduce the advantages of moving into management. ike9898 (talk) 19:21, 19 January 2010 (UTC)
- That may be true now, but think what will happen in the future. My guess is that in the long run you will be earning more money and having more status. 78.151.106.238 (talk) 20:38, 19 January 2010 (UTC)
- In my particular situation (US government), the difference in pay is small and and in my current situation as a researcher, I have a fair degree of autonomy. Both these factors reduce the advantages of moving into management. ike9898 (talk) 19:21, 19 January 2010 (UTC)
- If you like long meetings, presenting in front of groups, and having to nag deadbeats who are late to work and who slack off, then, by all means choose management. If you don't like those things and like to create things of value yourself, then choose the technical path. Technicians can pat themselves on the back and say, "I built that web site" or "I made that program." Managers aren't bad people. Some take joy in befriending their employees and rewarding them when they succeed. But eventually, you have to discipline them, too. That's not always a fun thing to do.--Drknkn (talk) 19:33, 19 January 2010 (UTC)
- Its just as easy to spin that the other way - technicians would tend to do more repetative work with less responsibility, hence more boredom and lack of fulfillment. There are many different styles and fads of management or (not the same thing) leadership - autocratic, consultative, etc etc. Take your pick. And you can build something without doing the labouring yourself - hence you build bigger and more impresive things. 78.151.106.238 (talk) 20:38, 19 January 2010 (UTC)
- Engineers are people who can do what they don't control. Managers are people who control what they can't do. Cuddlyable3 (talk) 20:39, 19 January 2010 (UTC)
- Its difficult to recommend a book without having more information on what kind of management you see yourself doing, and what aspect of management interests you. Could you supply more details please? 78.151.106.238 (talk) 20:49, 19 January 2010 (UTC)
- More details as requested:
- The most sensible and accessible type of management for me is management in government research agency. Such managers are involved in research in a sense, but they interact with the research indirectly, never hands-on.
- I'm not interested in management in particular, but I have the type of personality that I can get interested in also anything (e.g. editing the Wikipedia article on Ladies' Home Journal - I am a hetero male and do not read this magazine!)
- It seems to me that windows of opportunity to switch tracks are limited. I'm concerned that I might regret letting such a window pass me by.
- It seems that many older people, even older technical people, have less patience, interest and competence with new technology. Presumably, someday I'll be one of those people.
- ike9898 (talk) 21:11, 19 January 2010 (UTC)
- Thank you for assuaging natural worries about how reading too much might be influencing you, though that was TMI. Cuddlyable3 (talk) 12:03, 20 January 2010 (UTC)
- What?? ike9898 (talk) 14:35, 20 January 2010 (UTC)
- The small print comment is about your macho declaration about not reading a ladies' magazine. Some people would be shy about such things. Cuddlyable3 (talk) 16:49, 20 January 2010 (UTC)
- Oh, OK. I offered the information to make it clear that I can get interested in things I have no good reason to be interested in. ike9898 (talk) 17:17, 20 January 2010 (UTC)
- The small print comment is about your macho declaration about not reading a ladies' magazine. Some people would be shy about such things. Cuddlyable3 (talk) 16:49, 20 January 2010 (UTC)
- So now the question is, what sort of management do managers do in a government research agency? 78.149.139.201 (talk) 23:37, 19 January 2010 (UTC)
- What?? ike9898 (talk) 14:35, 20 January 2010 (UTC)
- More details as requested:
- It is difficult to give the reply requested as a) from what the OP has said the OP may have been offered a supervisory job or perhaps a project management role rather than an executive management role, b) there's plenty of management theory but nearly all the time managers never refer to it according to personal experience and Mintzberg, c) the stuff you read in textbooks will probably have little relation to what you actually do, d) there is an enormous amount of worthless garbage written about management or business, particulary popular American books I have to say. But, bearing that in mind you could have a look at Fayolism. Something to remember about Fayol is that the english version does not give enough or any emphasis to feedback and checking, both vital - interested to see that the article says this was due to a mistranslation of "checking" as "control" in English. The autocratic leadership style of that time is rightly far out of date. Do not mistake leadership for management - a common mistake. I've seen bad ignorant managers believe that (coercive) leadership is all that needs to done while the organisation falls to pieces because no management is being done. I remember reading this: Writers on Organizations by D.S. Pugh and David J. Hickson. I found this a few minutes ago www provenmodels dot com which gives details of many familiar thories, although it still makes the same mistake about Fayol. Wikipedia says its on its blacklist, do not know why, perhaps it is a Wikipedia rip-off, but at least it packages it in a nice way. This is mentioned in a couple of articles http://www.amazon.co.uk/Organization-Theory-Design-Understanding-Organizations/dp/0324422717/ref=sr_1_4?ie=UTF8 never read it myself. The reviewer Stan Felstead has reviewed a lot of management books http://www.amazon.co.uk/gp/cdp/member-reviews/A39KA7J326RUCV/ref=cm_cr_dp_auth_rev?ie=UTF8&sort_by=MostRecentReview Update: If you like history I've found Management Thought by Jayanta K Nanda, which can be read in full online in Google Books. I'd like to read it myself. 92.29.57.199 (talk) 16:05, 20 January 2010 (UTC)
- I see that Henry Mintzberg, perhaps the greatest living star of management theory, has recently had a book titled Managing published, in 2009. So although I've never read it myself, it would probably be worth reading. The first part of it that I read on Amazon mentions "evidence-based" books - unfortunately the majority of management books published are dross that is not evidence based. Rarely if ever do you find books that offer insights based on someone's personal experience without reference to academic publications - they are always garbage written in the first-person like a memoir and full of waffle. One thing that they never taught me in all my business training was how to handle the paperwork, and how important the mundane task of having a good up to date filing system is, so that the facts you need are readily at hand and quickly retrievable. 78.147.245.100 (talk) 11:59, 22 January 2010 (UTC)
Gaussian and SI Units of Charge
Hello everyone. Looking over pages for various units, I'm a little confused on how the relationship between coulombs and statcoulombs. Basically, if Coulomb's Law is defined differently in SI and gaussian units, the units on force must still be the same (mass length/time2). Setting the two forms equal to each other, I get a formula somewhat different to the one at the bottom of the statcoulomb page. I obtain
substituting in and throwing in a factor of for converting kilograms to grams and meters to centimeters, I get 1 statcoulomb = 2997924580 coulombs, ie the exact opposite of what the page says (and opposite everything that every other conversion table I've looked at said). What's wrong with my derivation? Thanks :) Bennybp (talk) 18:10, 19 January 2010 (UTC)
- If you have two numerical values a and b for the charge, in SI coulomb and statcoulomb respectively, and numerical values f and g in SI and Gaussian force units, and numerical values r and q in SI and Gaussian distance units, then the equation for two charges of the same size is
- Obviously, g = 105f and q = 102 r, so
- If b=1, then
- It seems like you forget that if you have a larger unit of charge, the numerical value for a given real charge is smaller ;-)
- Icek (talk) 06:51, 22 January 2010 (UTC)
- That makes sense. Looks like I need to crack open my high-school math book again. Thanks a bunch! --Bennybp (talk) 06:23, 24 January 2010 (UTC)
Relation between angular velocity and torque
What is torque formula of roatating equipment, if equipment rotate at constant angular velocity? —Preceding unsigned comment added by Mananmodi11 (talk • contribs) 18:24, 19 January 2010 (UTC)
- If the angular velocity is constant, the net torque is zero (just as if the net linear velocity is constant, the net force is zero). In real life, if there is a decelerating torque due to friction, that means you'll need to apply an equal and opposite torque to maintain the angular velocity. -- Coneslayer (talk) 18:30, 19 January 2010 (UTC)
- That is true for an isolated body. A rotating part of equipment that transmit power, such as a gearwheel in a clock or a car transmission, can have constant angular velocity while exerting a high torque. Cuddlyable3 (talk) 20:34, 19 January 2010 (UTC)
- What? It's the net torque on the "part of equipment" that then needs to be 0, not the torque it exerts on other things. Of course, there's an equal and opposite torque applied to it by the driven object, but there's also a torque applied to it by the "upstream" source (eventually by an off-center spring or gasoline explosion or…), and at constant angular velocity they add to 0. --Tardis (talk) 20:46, 19 January 2010 (UTC)
- Indeed. The car's transmission exerts a torque on the car's wheels and has a torque exerted on it by the car's engine. If the transmission has constant angular velocity, then those torques must cancel out. --Tango (talk) 01:06, 20 January 2010 (UTC)
- So if we couple a Bugatti Veyron engine to SteveBaker's Mini and break its gearbox (sorry SteveBaker), was the net torque on the gears zero? Cuddlyable3 (talk) 11:52, 20 January 2010 (UTC)
- Probably not. I've never tried connecting a gearbox to an engine too powerful for it, but I expect the gears would spin faster and faster until they broke. If their angular speed is increasing, there must be a non-zero net torque. --Tango (talk) 12:54, 20 January 2010 (UTC)
- The over-powered mini was being driven at constant speed up a steep hill when the errr...dysfunction manifested. That's my story anyway. Cuddlyable3 (talk) 16:42, 20 January 2010 (UTC)
- Probably not. I've never tried connecting a gearbox to an engine too powerful for it, but I expect the gears would spin faster and faster until they broke. If their angular speed is increasing, there must be a non-zero net torque. --Tango (talk) 12:54, 20 January 2010 (UTC)
- So if we couple a Bugatti Veyron engine to SteveBaker's Mini and break its gearbox (sorry SteveBaker), was the net torque on the gears zero? Cuddlyable3 (talk) 11:52, 20 January 2010 (UTC)
- Indeed. The car's transmission exerts a torque on the car's wheels and has a torque exerted on it by the car's engine. If the transmission has constant angular velocity, then those torques must cancel out. --Tango (talk) 01:06, 20 January 2010 (UTC)
- What? It's the net torque on the "part of equipment" that then needs to be 0, not the torque it exerts on other things. Of course, there's an equal and opposite torque applied to it by the driven object, but there's also a torque applied to it by the "upstream" source (eventually by an off-center spring or gasoline explosion or…), and at constant angular velocity they add to 0. --Tardis (talk) 20:46, 19 January 2010 (UTC)
- That is true for an isolated body. A rotating part of equipment that transmit power, such as a gearwheel in a clock or a car transmission, can have constant angular velocity while exerting a high torque. Cuddlyable3 (talk) 20:34, 19 January 2010 (UTC)
- The easiest thing is to think of Torque as being the rotational equivalent of Force in the linear realm. When you apply a net force to an object you get an acceleration - but in the real world, friction and drag provide opposing forces that increase with speed. At high enough speed, the friction and drag equal the propulsive force and the speed levels off. Same deal with torque - when you apply an unopposed torque, the object spins faster and faster - but eventually, friction and drag provide opposing torques that increase until you get a constant rotational velocity. SteveBaker (talk) 02:31, 20 January 2010 (UTC)
- :rolleyes:
- where rotational speed is in revolutions per unit time. See torque. --Heron (talk) 19:29, 20 January 2010 (UTC)
- I know that the OP said "constant angular velocity", but, to expand on SteveBaker's comment and answer the question implied in the heading, when the torque is unapposed it is equal to rate of change of angular momentum i.e. torque = moment of inertia times rate of change of rotational speed. (assuming a fixed axis of rotation) Dbfirs 17:57, 22 January 2010 (UTC)
The path of the moon through the sky
Would the path of the moon through the sky, from moon-rise to moon-set, always be part of a circle if viewed through a very large sheet of glass that was set vertical and parallel to the imaginary line from moonrise azimuth to moonset azimuth? I mean, an arc of a circle and not some other curve. 78.151.106.238 (talk) 20:32, 19 January 2010 (UTC)
- Almost entirely, yes. It is based on the rotation of the Earth and the current tilt of your view in relation to the moon. It is slightly (very slightly) skewed because the moon is also orbiting the Earth. It isn't enough that you'd really notice over a single night. -- kainaw™ 21:37, 19 January 2010 (UTC)
- No, it would be part of an ellipse, I think. It is a circle on the sky, but when you project it onto a plane (the glass) you'll get an ellipse unless your eye, the glass and the moon are lined up just right. You've mentioned where the glass is with respect the moon, but not where your eye is. --Tango (talk) 01:14, 20 January 2010 (UTC)
- No -- and it's not even "a circle on the sky." While above (or below) the horizon, the moon moves forward in celestial longitude and right ascension. Between rising and setting, the movement is enough to make its path vary significantly from a segment of a circle. Only an outer planet at stationary retrograde/direct (or a star) would closelt approximate a segment of a circle; and while the inner planets at stationary would move enough to distort a circle, the distortion would be small enough that "circle" would be a reasonable term. But the moon moves too fast, and is never stationary. (The exception would be when it is above or below the horizon very briefly, as it would be during certain times of the month in high terrestrial latitudes.)
unlikeliest thing to happen that did in fact happen? (that we know of)
Hi,
"Of all the things that were unlikely to happen, but did anyway, which one had been the unlikeliest?" (that we know of)
is my question even meaningful, or does it misconstrue statistics? (this is not homework). Thanks. 84.153.196.174 (talk) 20:55, 19 January 2010 (UTC)
- That your particular sperm fertilized the egg, against compitition from millions or perhaps billions of other sperm. That is probably the most unlikely thing in anyones life. 78.151.106.238 (talk) 20:58, 19 January 2010 (UTC)

- No, it's the most certain thing in anyone's life. Cuddlyable3 (talk) 11:43, 20 January 2010 (UTC)
- Anything after the event has taken place is the most certain thing, no matter how extremely unlikely it was before the event. 92.24.85.238 (talk) 11:41, 21 January 2010 (UTC)
- Don't all sperm have the exact same DNA? What makes this not a distinction without a difference? 84.153.196.174 (talk) 21:00, 19 January 2010 (UTC)
- No. They each have a different (overlapping) half of the source's DNA. --Tardis (talk) 21:04, 19 January 2010 (UTC)
- No, it's the most certain thing in anyone's life. Cuddlyable3 (talk) 11:43, 20 January 2010 (UTC)
- What humans naturally consider "unlikely" is actually the most likely. For example, coincidence is considered unlikely, but high levels of coincidence are statistically very likely. I used this concept in a utterly pathetic novel I worked on for many years in which it was easy to identify when future people attempted to manipulate the past because they tended to remove coincidence and randomness. I used an example of asking a person to arrange blocks in a random manner to make it appear that the blocks simply fell out of a box. There will always be clear statistical identifiers when the humans arrange the blocks "randomly" and when they are actually just dumped from a box.
- So, what is the purpose of this rambling nonsense? The most unlikeliest thing would be the complete absence of anything we consider unlikely since unlikely things happen all the time. -- kainaw™ 21:15, 19 January 2010 (UTC)
- Humans, as opposed to physicists..? There's something funny about this, anyway. In what you call human terms, to be worth mentioning, unlikely things must be both unlikely and significant. Mere randomness like a particular fingerprint or Feynman's numberplate (mentioned below) don't count. The unlikely events which really count are the ones which contain knowledge, and knowledge is what probability tends towards (convergent evolution of ideas or genes). So what we call unlikely is finely balanced between the probable and improbable, and "most unlikely" doesn't make much sense because it's all a bit subjective; context matters, the context of type of events to include. 81.131.36.185 (talk) 12:21, 20 January 2010 (UTC)
- One runs into trouble with this kind of thing quickly because the odds of specific things happening are often low but the odds of general things happening are high. For example: starting with the initial set of conditions shortly after the Big Bang, what are the odds that some of the matter would coalesce into our Moon, and some of the other matter would coalesce into an asteroid, and that asteroid would smack into our Moon? Yet, the odds that moons will form, and that asteroids will form, and some asteroids will hit some of those moons, are quite high. Delineating the scope of the analysis is really rather important, or else you end up with ridiculous (and meaningless) answers. --Mr.98 (talk) 21:19, 19 January 2010 (UTC)
- Peel an orange and look closely at the patterns the fibres make on the surface of the segments. What were the chances of exactly that pattern occurring? So small as to be virtually impossible. And yet it happened. The closer you look at something, the unlikelier it was. Or as Terry Pratchett put it: 'Scientists have calculated that the chance of anything so patently absurd actually existing are millions to one. But magicians have calculated that million-to-one chances crop up nine times out of ten.' Neurotip (talk) 21:52, 19 January 2010 (UTC)
- The chances of that particular pattern emerging are very small. But the chance of some pattern emerging is 100 percent. It's kind of like with the lottery. The odds of you winning it are very small. But the odds of someone winning it are very high. ←Baseball Bugs What's up, Doc? carrots→ 15:51, 20 January 2010 (UTC)
You know, the most amazing thing happened to me tonight. I was coming here, on the way to the lecture, and I came in through the parking lot. And you won't believe what happened. I saw a car with the license plate ARW 357. Can you imagine? Of all the millions of license plates in the state, what was the chance that I would see that particular one tonight? Amazing! - Richard Feynman
- Law of Truly Large Numbers may also be relevant. --Mark PEA (talk) 22:27, 19 January 2010 (UTC)
It's an interesting question. If the energy of the universe is finite, then the number of possible quantum states of the universe is finite, so there must be a nonzero minimum probability for any possible state -- but I wouldn't begin to know how to estimate it. Looie496 (talk) 00:11, 20 January 2010 (UTC)
- Yes, but there is no point looking at the probability of the universe being in a particular state. You need to look at the probability of the universe being in one of a set of states. We don't care, for example, if the oxygen molecule one metre to my left were actually 1.01 metres to my left. This is related to the concept of entropy. Not taking this into account leads to a lot of false claims about the unlikelyness of events. Imagine if I were to take a well shuffled deck and deal two cards face up and it turns out to be 2 7's. You may think that was unlikely and work out that the odds of getting those two cards was 1/52*1/51, or 0.04%, which is extremely unlikely and we may suspect that the deck wasn't properly shuffled. You should, however, note that it doesn't matter what order the cards came down in, so you should double the probability. You should also note that it doesn't matter what suits the 7's are, so you should multiply the probability by 6. You should then note that it doesn't actually matter that they were 7's, you would have been just as surprised by any pair, so you should multiply the probability by 13. Once you do all that you are left with the probability of two randomly chosen playing cards being a pair, which is 5.88%. Far higher than the 0.04% we started with. The chance of getting a pair is actually better than 1 in 20. It isn't very unlikely at all, so we shouldn't have been that surprised. The reason we were was because we were distinguishing between outcomes that were actually the same for our purposes. --Tango (talk) 01:03, 20 January 2010 (UTC)
- If you apply the Bekenstein bound to the observable universe (which may or may not make sense) then it would argue that the universe has no more than 2(10124) possible configurations given its current size. Of course that is an incomprehensibly large number. Dragons flight (talk) 01:05, 20 January 2010 (UTC)
- Any event that just happened is the most improbable. If you take the big bang as a certainty - then the probability of a particular subatomic particle being at a particular place a picosecond later is less probable - the probability of some other subatomic particle being at some other place a second later is less probable still. The longer you go out in time, the less probable any given thing is.
- The probability that I just flipped a coin and it came up heads is so astronomically improbable that at the instant I did it, that was the least probable thing that's ever happened in the entire history of the universe (apart from the uncountable number of other things that happened at that precise instant). For that coin to come up heads, I had to happen to pick up that coin at that 7/11 store because I happened to run out of milk because my son happened to have visited because...(time passes)...humans happened to evolve because...(more time passes)...the first self-replicating molecule appeared because...(because, because, because)...because that subatomic particle was here a picosecond after the big bang and not a bazillionth of a meter to the left.
- But this is a kinda useless conclusion. What we generally mean when we say "What are the odds that this coin will come up heads?" is more like "Given that this coin is here at all and given that there is a sentient being present to toss it and observe the results...what are the odds that it'll come up heads?" - which reduces the unimaginably long odds to something closer to 50/50.
- So the question we're being asked fails to allow a reasonable answer because it doesn't specify enough preconditions that we're allowed to assume. What is the probability of a coin coming up heads given that coins exist and people exist to toss them is a really high probability. The probability of a coin coming up heads given the existence of life on earth is a much lower probability and the probability given only that galaxies formed in the early universe is much MUCH lower still.
- Hence, there simply isn't a good answer - and we shouldn't pretend otherwise. SteveBaker (talk) 13:40, 20 January 2010 (UTC)
- Agreed - the problem stems from observer bias. Humans perceive randomness when there is in fact order; and they perceive order from systems which are statistically spread or indeterminate. Short of an elaborate mathematical definition for certain randomness measures, like deviations from expected values, or spectrum of those deviations (ad nauseum, deviations from expected deviations from expected deviations...), "degree of randomness" is poorly defined. High entropy is often equated with randomness - but this is a mistake of plain english language that doesn't really mean the same as scientific language. As alluded to in the Hitchhiker's Guide to the Galaxy, a really hot cup of tea is awfully high entropy - but it's not really very "unlikely" as far as human perception goes. In truth, it's because our limited capability to perceive certain aspects of the tea, like the individual motions of the billions and billions of individual tea molecules. If we could perceive and conceive those concepts - in actuality and not in abstraction - then we would be blown away every time we looked at a gust of wind or a warm fluid or even a solid object. How unlikely that Molecule #46983743 has "chosen" to collide with molecule #3298210932! What's the probabilty! The Dick Feynman quote really hits this point hard - who cares if an event is random if it is insignificant? But that's really just restating that we evaluate probabilities through the bias of being a human observer - with human preconceptions about what is "signficant." Nimur (talk) 17:32, 20 January 2010 (UTC)
- Hence, there simply isn't a good answer - and we shouldn't pretend otherwise. SteveBaker (talk) 13:40, 20 January 2010 (UTC)
So could things be a bit simpler...What is the highest-odds (major) sports betting outcome? (E.g. a 15000/1 horse romping home to victory in the Grand National?). 194.221.133.226 (talk) 15:35, 20 January 2010 (UTC)
- It's more probable than a certifiably dead horse winning the race. ←Baseball Bugs What's up, Doc? carrots→ 15:49, 20 January 2010 (UTC)
- Like most things, the probablility isn't zero. Consider a prize-winning racehorse, running at the limits of it's ability gets clear of the pack - suffers a sudden heart failure 5 feet from the tape - momentum propels it to victory (of a sort). SteveBaker (talk) 00:03, 21 January 2010 (UTC)
- Well, the Grand National article lists the longest shot winners at 100 to 1, the same as Man o' War's only loss (to Upset, fittingly enough). Buster Douglas vs. Mike Tyson was only about 45 to 1. Clarityfiend (talk) 00:37, 21 January 2010 (UTC)
- Greece won Euro 2004 as a 150-1 underdog, according to the BBC. Clarityfiend (talk) 02:53, 21 January 2010 (UTC)
The probability of everything that has occurred is one. Just because proscribed probability might put the chance of something happening at some fraction of one, that estimate is disproved when the event occurs. In short, statistics is a load of rubbish. Useful rubbish, but it it makes no contact whatsoever with the real world. Vranak (talk) 19:58, 21 January 2010 (UTC)
Go back to the question: "Of all the things that were unlikely to happen, but did anyway, which one had been the unlikeliest? (that we know of)." So: 1) things, 2) that happened. The answer would have to be something that has happened only once, and in the longest period of time. The answer is: The big bang. By definition, for anyone to ask this question, the big bang -- or some other creation event -- would have had to have occurred, but that doesn't change the fact "(that we know of)" that the big band has occurred only once in all the history of the universe, making it the most unlikely event to happen "but did anyway." Every other event that has happened has either happened more than once or more recently.
escaping black holes
Is it correct that a virtual graviton (gravity carrier) can escape out of black hole, whereas a real or actual graviton (gravity wave carrier) cannot?? 89.138.153.164 (talk) 21:00, 19 January 2010 (UTC)
- Perhaps. --Neptunerover (talk) 02:07, 20 January 2010 (UTC)
- I think that can be paraphrased as how can one tell? It's true on the straightforward model of a black hole as a gravity well but who knows what model will end up being accepted. I'm pretty certain we'll never be able to do an experiment to find out! See Hawking radiation for this general area, there's no reason to suppose gravitons would act any differently. Dmcq (talk) 10:55, 20 January 2010 (UTC)
Resistance/Stress
Does the resistance of a wire vary when the stress is changed, and is there a formula to relate the 2? Various circuits I've been building with potential dividers have seemed a bit odd and I was wondering whether the stress of the wire could affect it's resistance. Harland1 (t/c) 21:40, 19 January 2010 (UTC)
- Yes, the stress on a wire causes strain which changes the length of the wire, and resistance varies with length. (A strain gauge works on this principle. By measuring the change in resistance of the strain gauge it is possible to determine the strain of the component to which the gauge is attached. Using knowledge of the elastic modulus of the material it is then possible to determine the stress experienced by the component.) Dolphin51 (talk) 21:50, 19 January 2010 (UTC)
- There is a formula that relates the resistance R of a piece of wire of length L, the resistivity of the metal, and the sectional area A of the wire. See Resistivity#Explanation. Dolphin51 (talk) 11:18, 20 January 2010 (UTC)
- Note that under strain, the resistivity may not be isotropic (the same in all directions) anymore: together with its inverse, the conductivity (see that article), it becomes tensorial. Effectively, numbers become matrices. — Pt (T) 18:18, 21 January 2010 (UTC)
Relationship between moon phase and the azimuths of moonrise or moonset
Do moons with the same phase have the same azimuth of moonrise or moonset? And in this diagram here, http://hal.physast.uga.edu/~jss/1010/ch2/02-21.JPG it appears that a new moon rises higher in the sky than a full moon. Is that correct? I assume that the information given in the diagram applies to any time of year and any location (more or less) in the northern hemisphere. 78.151.106.238 (talk) 21:57, 19 January 2010 (UTC)
- Since the moon's orbit is close to the Earth's orbital plane, and since the azimuth is measured with respect to the Earth's North Pole, I would assume it depends strongly on the season. Dragons flight (talk) 22:10, 19 January 2010 (UTC)
- It looks to me as though the diagram applies to someone on the equator at the time of one of the equinoxes. Presumably they have done this for simplicity. The season and latitude of the observer makes a huge difference to azimuth as well as the rising and setting times of the moon. The new moon in winter will culminate low in the sky; the full moon in winter will culminate high in the sky, and vice versa.--Shantavira|feed me 10:18, 20 January 2010 (UTC)
- The moon's phase has nothing to do with the apparent position of the moon-in-itself -- it's a combination of the positions of the moon AND the sun. Therefore, the moon's apparent position is not unilaterally equatable with the moon's phase. Answer to your question: No.
Is a table available anywhere of the daily azimunths, ideally with the phase as well? 92.29.130.174 (talk) 12:09, 23 January 2010 (UTC)
gene concepts
- Question removed: See talk page SteveBaker (talk) 04:56, 20 January 2010 (UTC)
EADS Astrium orbital power
Why exactly wouldn't it be dangerous [6] to be hit from orbit by 20 kilowatts of infrared laser beam? 81.131.45.171 (talk) 22:43, 19 January 2010 (UTC)
- Probably because the beam would be quite spread out by the time it reached the ground. I can't find any details, though. --Tango (talk) 23:49, 19 January 2010 (UTC)
- Per Tango, I don't know the details of the beam in question but it almost certainly has to do with the spreading of the beam by the time it reaches Earth's surface. Bright sunlight deposits roughly 1 kilowatt (kW) per square meter on the Earth's surface. If the beam is spread out over at least twenty square meters (a disc about 2.5 meters, or about eight feet, across) then the beam is no more intense than sunlight. That's not going to cook much. TenOfAllTrades(talk) 00:33, 20 January 2010 (UTC)
- It'll be safe at this experimental scale - but a serious multi-megawatt setup could certainly cook if you got in the way of it, so you'd have to take precautions in the event that the satellite lost stability or something. Aside from simply radioing commands to the satellite in the event of problems with the incoming beam, I'd place a mirror on the ground detector to reflect a tiny fraction of the laser power back to the satellite. The satellite would be designed to cut off the beam if it doesn't "see" it's own reflection in that mirror. Ideally you'd want the cutoff to be a passive system - if the satellite doesn't actively assert that the beam is on-target, it's shut off automatically. That way if it drifts off-target, the power gets cut off even if the ground station goes AWOL or the satellite has lost on-board power - or both. SteveBaker (talk) 01:46, 20 January 2010 (UTC)
- Good God yes. Something like this, it definitely needs to be fail safe. Apart from the judgement of the satellite's systems, based on reflections it can see, I'd have it sent regular radio messages saying "keep going", so that a change or halt in the radio messages (secure and encrypted from specific sites) would cause it to stop immediately. Not just ideally: I wouldn't sign off on a deadly thing like that up without fail safe systems. If it has to be told to stop in the event of failure, that's a recipe for disaster. 86.178.230.208 (talk) 17:48, 20 January 2010 (UTC)
- That mirror should be a Corner reflector to avoid a tricky aiming problem. Cuddlyable3 (talk) 11:30, 20 January 2010 (UTC)
- A multi-megawatt infrared laser would probably be even more spread out - if it was spread out to a disc a little over 100m in diameter (which sounds plausible to me) then it would still be no brighter than the sun. --Tango (talk) 04:10, 20 January 2010 (UTC)
- Then what would be the point of it? Why not just stick some solar panels out there? That doesn't make sense. SteveBaker (talk) 04:38, 20 January 2010 (UTC)
- Night. Cuddlyable3 (talk) 11:31, 20 January 2010 (UTC)
- The 1kW/m2 figure is a maximum. You only get it at noon near the equator. With a laser beaming the energy from space you get it 24/7 anywhere you want. But basically you are sticking solar panels out there - that's what the receiving station is, a big array of solar panels (optimised for the frequency of the laser, presumably). --Tango (talk) 07:21, 20 January 2010 (UTC)
- I'm a bit puzzled by how a satellite can stay out of the earth's shadow 24/7 when the moon doesn't (but not entirely unbelieving). 81.131.36.185 (talk) 12:25, 20 January 2010 (UTC)
- You put it at an appropriate Lagrange point. You may well need relay satellites to reach a receiver 24/7, though, since lasers do require line of sight. An alternative is to just put it in a geostationary orbit and accept that it will be in shadow for a short period occasionally. According to this source, that period is at most 70 minutes and is no time at all for most of the year (it's only near the equinoxes that they pass through the Earth's shadow). --Tango (talk) 12:51, 20 January 2010 (UTC)
- I'm a bit puzzled by how a satellite can stay out of the earth's shadow 24/7 when the moon doesn't (but not entirely unbelieving). 81.131.36.185 (talk) 12:25, 20 January 2010 (UTC)
- The 1kW/m2 figure is a maximum. You only get it at noon near the equator. With a laser beaming the energy from space you get it 24/7 anywhere you want. But basically you are sticking solar panels out there - that's what the receiving station is, a big array of solar panels (optimised for the frequency of the laser, presumably). --Tango (talk) 07:21, 20 January 2010 (UTC)
- Night. Cuddlyable3 (talk) 11:31, 20 January 2010 (UTC)
- Then what would be the point of it? Why not just stick some solar panels out there? That doesn't make sense. SteveBaker (talk) 04:38, 20 January 2010 (UTC)
- A multi-megawatt infrared laser would probably be even more spread out - if it was spread out to a disc a little over 100m in diameter (which sounds plausible to me) then it would still be no brighter than the sun. --Tango (talk) 04:10, 20 January 2010 (UTC)
- That mirror should be a Corner reflector to avoid a tricky aiming problem. Cuddlyable3 (talk) 11:30, 20 January 2010 (UTC)
- You can also use a less-exotic sun-synchronous orbit. --Tardis (talk) 15:48, 20 January 2010 (UTC)
- Why would that help? --Tango (talk) 21:14, 20 January 2010 (UTC)
- Something in a dawn-dusk orbit (see the article) always sees the sun. --Tardis (talk) 15:42, 21 January 2010 (UTC)
- Why would that help? --Tango (talk) 21:14, 20 January 2010 (UTC)
- You can also use a less-exotic sun-synchronous orbit. --Tardis (talk) 15:48, 20 January 2010 (UTC)
- That still doesn't make sense. If the beam is really no brighter than the sun, then why not just take the money you were going to spend on getting a spaceship to a Lagrange point and make two solar panels, that way you've don't have to worry about nighttime because panel #2 saved it up to a battery. APL (talk) 04:57, 21 January 2010 (UTC)
- Yes, exactly. Large-scale storage of electricity is difficult - but nothing like as difficult as a spaceship! I thought the plan was (ultimately) to launch a few square kilometers of solar collecters to the Lagrange point and beam gigawatts of power down to a relatively small earth-based receiver. If you can only get a few times brighter than the sun, then there seems very little point in doing this. SteveBaker (talk) 14:31, 21 January 2010 (UTC)
- Well, I don't know what the maximum safe level would be. You can probably have more IR than you can sunlight, since it is the UV from the sun that does most of the damage to humans. Really high power transmission would be great, but I would be surprised if it ever got planning permission. Most plans I've seen have stressed that the beam would be harmless even if it went off-target. Even with a 1kW/m2 beam you would get several times what you would from the sun. You can get a more efficient receiver due to it working over a narrower range of wavelengths and you get the beam constantly at full power with very few breaks (depending on the orbit of choice - I think geostationary is my favourite, which has a few short breaks around the equinoxes). --Tango (talk) 17:59, 21 January 2010 (UTC)
- Yes, exactly. Large-scale storage of electricity is difficult - but nothing like as difficult as a spaceship! I thought the plan was (ultimately) to launch a few square kilometers of solar collecters to the Lagrange point and beam gigawatts of power down to a relatively small earth-based receiver. If you can only get a few times brighter than the sun, then there seems very little point in doing this. SteveBaker (talk) 14:31, 21 January 2010 (UTC)
- Just noticed this, I'm surprised no one made a Sim City 2000/Sim City 3000 reference with their satellite microwave power plants which could of course misfire and obliterate much if your city. Looking at the SC2k article also pointed me at Space-based solar power which no one has mentioned and provides some info on things like targetting systems although fairly limited info on lasers and nothing on this particular proposal. Incidentally while only of minor relevance since the numbers above were just guesstimates, if the satellite is transmitting 10-20kW you're not going to receive that on the surface of earth. According to the talk page, you may lose about 1/3. Nil Einne (talk) 00:32, 20 February 2010 (UTC)
Where do Squirrels go in the Winter?
Also, why does DC have black squirrels? —Preceding unsigned comment added by 199.172.169.21 (talk) 23:29, 19 January 2010 (UTC)
- Do they go anywhere? We have squirrels all year round, and the article seems to indicate that they build fairly permenent dens. As for black squirrels, check out their article. They're just a subgroup of the Eastern Gray Squirrel, and can occur wherever gray squirrels are found, though have larger populations in certain areas, including Washington DC. The article says they were introduced in the early 1900's at the Smithsonian National Zoological Park. Buddy431 (talk) 23:51, 19 January 2010 (UTC)
- Squirrels don't go anywhere. They are well known for hiding nuts for the winter - they wouldn't do that if they weren't staying for the winter. --Tango (talk) 00:31, 20 January 2010 (UTC)
- I don't believe squirrels migrate nor hibernate. I've seen them in the mountains all year round. --Neptunerover (talk) 00:52, 20 January 2010 (UTC)
- Likewise in the city, here in Toronto. Actually, what they like to do in winter is break into the attic of my house... --Anonymous, 01:54 UTC, January 20/10.
- I used to live in the Lower East Side of Manhattan, and on the way home from elementary school down the FDR Drive, I would often see a bunch of black eastern gray squirrels in the lawn of the Peter Cooper Housing. DRosenbach (Talk | Contribs) 02:54, 20 January 2010 (UTC)
- It is utterly untrue that black squirrels are a phase of Gray Squirrels. In my town, we have gray squirrels. The next town has black squirrels. They do not apparently interbreed. Black squirrels do not grow up to be gray squirrels, nor conversely. Someone should do the genetic analysis and confirm the disparity. Edison (talk) 05:17, 20 January 2010 (UTC)
- You might want to read the article on the Black squirrel. ←Baseball Bugs What's up, Doc? carrots→ 05:58, 20 January 2010 (UTC)
- What's utterly untrue is that it's utterly untrue. Maybe you haven't seen them breed because you were barking up the wrong tree. DRosenbach (Talk | Contribs) 14:24, 20 January 2010 (UTC)
- There are a few black squirrels around here, and all of our squirrels are Red Squirrels, not Grey Squirrels. Googlemeister (talk) 15:34, 20 January 2010 (UTC)
- What's utterly untrue is that it's utterly untrue. Maybe you haven't seen them breed because you were barking up the wrong tree. DRosenbach (Talk | Contribs) 14:24, 20 January 2010 (UTC)
- I'm curious how you're so adamant on the mating practices of local squirrels? How much squirrel mating do you have to observe before you can make confident proclamations on the habits and prejudices of the entire local squirrel population?
- Besides, even if you're right that they don't interbreed, that's not a solid indication that they're incapable of it. Perhaps if squirrel 'tail' is plentiful (Get it? Get it?) they just stick to their own. APL (talk) 15:39, 20 January 2010 (UTC)
- We have the article about the black squirrel vs. the personal observations (a.k.a. "original research") of one user. That doesn't say which is right. But it does suggest that he needs to find some citations. :) ←Baseball Bugs What's up, Doc? carrots→ 15:46, 20 January 2010 (UTC)
- Then again, when I look at the tree squirrel article, it becomes clear that North America has many types of similar looking squirrels with overlapping ranges. It's entirely possible that the "gray squirrels" edison is refering to are not in fact Eastern Gray Squirrels, in which case they are truly different (though closely related) species. Buddy431 (talk) 21:15, 20 January 2010 (UTC)
- We have the article about the black squirrel vs. the personal observations (a.k.a. "original research") of one user. That doesn't say which is right. But it does suggest that he needs to find some citations. :) ←Baseball Bugs What's up, Doc? carrots→ 15:46, 20 January 2010 (UTC)
- You might want to read the article on the Black squirrel. ←Baseball Bugs What's up, Doc? carrots→ 05:58, 20 January 2010 (UTC)
- Likewise in the city, here in Toronto. Actually, what they like to do in winter is break into the attic of my house... --Anonymous, 01:54 UTC, January 20/10.
It's weird, I was just talking with my friend about this yesterday. I have a further question - where do squirrels sleep when it's not winter? Do they all have nests or something somewhere? Do they just nestle in some big coniferous somewhere? Do they just sleep sitting on a branch? What do they do when a basement or attic is not available to them? TastyCakes (talk) 15:53, 20 January 2010 (UTC)
- Again, read the article: eastern gray squirrel. They build nests known as dreys, in trees or other suitable places (like attics). Depending on where you live, you may be thinking of a different type of squirrel, in which case you'll have to read the appropriate article.Buddy431 (talk) 16:06, 20 January 2010 (UTC)
- Yes -- here's a close-up photo. Similar nests can be seen throughout NYC and northern Jersey (other places as well, but I try not to leave the area and sometimes even feign ignorance that other places even exist :) DRosenbach (Talk | Contribs) 04:26, 21 January 2010 (UTC)
- I can accept the black squirrels being a "subgroup" of the more common type, as our article says, but elsewhere I have seen the unlikely claim that the dark fur is a "phase" of the lighter squirrel. I took this statement to mean a "growth phase," with black squirrels later becoming gray squirrels or vice versa. Or does it mean a stable fur color? Since I live near a boundary between the ranges of the two groups, I see black squirrels to the north and the more common type to the south. It is rare for a black one to cross over the boundary zone. One block may have 3 gray ones and no black ones out foraging in one town, and conversely in the next town. There could be interbreeding with mixed litters of black ones and gray ones, but we do not see blended colors (intermediate shades). In many years of observation, I have never seen them hanging around together. A particular street is the approximate borderline, stable over many years plus or minus a block or two. The black squirrels might flourish more in more heavily wooded areas. Some genetic testing would be informative. The black squirrels in different regions need not be the same genetic subtype. There are also towns with white squirrels. In some cases, such as Kenton, Tennessee the presence of white squirrels is a point of pride for the town and active measures are taken to protect them and keep others out. Apparently not all white squirrel populations are albinos. As for where they go in winter, they are fond of gnawing their way into attics. Others insulate nests or pockets in dead trees with leaves and survive there, then come out to eat some snow and raid bird feeders and garbage cans of the plastic variety. Some refer to them as "tree rats." Chipmunks of "ground squirrels" hibernate, but gray squirrels don't. Edison (talk) 17:59, 20 January 2010 (UTC)
- "Phase" is used in place of "race" or "subspecies", especially where the subgroup isn't geographically or otherwise isolated. It's pretty much equivalent to the word "breed", which is normally only used in domesticated species. Matt Deres (talk) 21:28, 20 January 2010 (UTC)
- Black and gray eastern gray squirrels being the same species doesn't imply there would be shades in between. Some traits are either on or off, for example when the trait is determined by a single gene. It also doesn't imply that all populations of eastern gray squirrels would have similar distributions of gray and black. If the gene pool in one area has a lot of black squirrels, that's not likely to change over time unless there's selective pressure. And the selective pressures might be different in different areas, since as the article says the black ones are more successful in dark woods. Rckrone (talk) 21:49, 20 January 2010 (UTC)
- All the black squirrels I've ever seen had tufted ears. There were gray squirrels (w/ white bellies) around too, but none of the different types (there were even red 'downtown' squirrels) seemed to associate with each other at all. --Neptunerover (talk) 16:10, 21 January 2010 (UTC)
January 20
freckles
- Question removed: See talk page. SteveBaker (talk) 04:55, 20 January 2010 (UTC)
- can't see it on the talk page? --TammyMoet (talk) 10:58, 20 January 2010 (UTC)
- Sorry: look at 71.100 is back and 71.100 is back (again). SteveBaker (talk) 13:24, 20 January 2010 (UTC)
- Ah! Thank you. --TammyMoet (talk) 15:35, 20 January 2010 (UTC)
Ohm's Law
1) Ohm's law says that J is proportional to E...but is this E the E field created by the battery, or something else? Because when this expression is manipulated to get V=IR, this V is said to be the voltage drop caused by the resistor, which would seem to suggest that the E is not the electric field due to the battery. But then what is it?
2) When a current passes through a resistor, the electrons lose energy. Wouldn't this mean that, due to the reduced kinetic energy of the electrons, they would travel less quickly and thus the current intensity would drop? Why does the current stay the same? —Preceding unsigned comment added by 173.179.59.66 (talk) 02:59, 20 January 2010 (UTC)
- The only difference between these forms of Ohm's Law is that one divides both sides of the equation by unit-length. As far as whether the voltage drop is due to the resistor or due to the battery... well, don't get stuck on what causes the voltage drop. The voltage drop merely exists (because there is a potential energy difference between the battery terminals, caused by a chemical reaction). And the voltage drop must exist somewhere - that somewhere happens to be across the resistance. Whether the voltage drop is caused by the resistor or by the battery is semantic - the voltage is a potential difference between the battery terminals, and it exists across the terminals of the resistor. As far as the kinetic energy detail, the assumption of a linear ohmic material (like a resistor) is that the electric potential energy of the electrons is not related to the kinetic energy of their drift velocity. Specifically, drift velocity is proportional to the applied electric field. This necessarily means that the kinetic energy of the electron drift velocity is not equivalent to the electric potential drop. (Rather, the kinetic energy due to electron drift motion is a tiny fraction of the total energy that the electron gains from the electric potential field). The electric potential energy manifests in other ways, including thermal motions and interactions with the conductor's atomic lattice. Mostly, though, it is potential energy, not kinetic energy. See electrical conductivity, and electron mobility, for more of the physics related to this principle. Nimur (talk) 03:35, 20 January 2010 (UTC)
- Another and less eloquent perspective: Things that are equal are equal. The drop of potential across the resistor MUST equal the rise of potential across the battery, or the terminals (nodes) would have two different voltages at the same time. There is internal resistance in a practical battery, which led some scientists to claim that Ohm's Law failed, back in the day. Many materials do not follow Ohm's Law, including semiconductor devices and vacuum tubes, as well as light bulb filament, whose resistance changes with the temperature. Edison (talk) 05:14, 20 January 2010 (UTC)
- 1) E is the potential difference (= its voltage V) created by a battery. It can be communicated from place to place by conductors. Where there is no conductor E exists as a field but field is a concept that is useful here only in a homogenous environment without localised conductors, such as inside a material or in air or vacuum. Resistors do not cause any voltage by themselves; if they did then practically every object except a very few that are superconductors or perfect insulators (exist?) would produce a voltage. V=IR means a current I flowing in a resistance R causes a voltage V to appear across the resistor or a voltage V applied across a resistor causes a current I to flow. Which viewpoint you choose doesn't matter because they are just rearrangements of the same interdependance.
- 2) The E field gradient inside a simple resistor is linear so the drift velocity of electrons in the direction of the field is constant throughout the resistor material. Electrons lose their potential energy to the material, this causes Joule heating. Their velocity and the current intensity do not change until the current passes out of the resistor into a material of different resistance.
Ah okay...but if the V manifests in something abstract like a change in potential energy, then how is a voltmeter able to measure it? —Preceding unsigned comment added by 173.179.59.66 (talk) 12:24, 20 January 2010 (UTC)
Wait let me guess, does the E field create a magnetic field which is then measured? —Preceding unsigned comment added by 173.179.59.66 (talk) 12:28, 20 January 2010 (UTC)
- Whenever using a voltimeter to measure the voltage you are actually placing a resistor that lets an small current through. (small enough that it won't disturb the behaviour of the circuit). By measuring this circuit the voltimeter can tell what's the voltage. Dauto (talk) 14:42, 20 January 2010 (UTC)
And what happens in the space between resistors? Let's say two resistors are connected in series. Then there is a V drop through the first resistor, and another V drop through the second resistor, but between the two resistors the voltage doesn't change (or negligibly so). I would guess that this implies that the E field is practically 0 in this area...so how does E field created by the battery dissapear here? Do the charges like rearrange to create a 0 E field? —Preceding unsigned comment added by 173.179.59.66 (talk) 12:37, 20 January 2010 (UTC)
- Yes, the charges at the edge of the resistor rearrange themselves accordingly. Dauto (talk) 14:42, 20 January 2010 (UTC)
Was a study ever done suggesting what might have happened had the leak in Challenger's SRB field joint been directed outward instead of toward the ET?
Chapter III: The Accident of the Rogers Commission Report details the timeline of events, with flame from the leak first visible at at +60s. During the next four or five seconds, before the LH2 tank was breached, the craft's control system started to react to the forces caused by the plume and the reduced right booster chamber pressure. The spacecraft broke apart at +72s, but both boosters continued to burn until they were detonated by the RSO at +110s, only 10 seconds before normal burnout. Was there any chance that the Challenger could have survived had the flame plume not been directed at the ET? 124.157.247.221 (talk) 06:25, 20 January 2010 (UTC)
- Shuttle - Key Dates: January 24, 1985 The shuttle suffered from blow-by events of the O-ring several times during launches before the disaster. The engines compensated for the slight change in pressures. Zzubnik (talk) 10:11 January 20 2010 (UTC)
- Yes - this kind of event had happened many times before - but it was only when the blowout hit something important that it caused the mission to fail. Sadly, the previous occasions were only spotted in hindsight after the Challenger disaster. SteveBaker (talk) 13:22, 20 January 2010 (UTC)
- Your last sentence is untrue, according to our article STS-51-C and according to Zzubnik's link: "The shuttle flight 51-C of January 24, 1985, was launched during some of the coldest weather in Florida history. Upon examination of the booster joints, engineers at Thiokol noticed black soot and grease on the outside of the booster casing, caused by actual gas blow-by. This prompted Thiokol to study the effects of O-ring resiliency at low temperatures. They conducted laboratory tests of O-ring compression and resiliency between 50lF and 100lF. In July 1985, Morton Thiokol ordered new steel billets which would be used for a redesigned case field joint. At the time of the accident, these new billets were not ready for Thiokol, because they take many months to manufacture." Comet Tuttle (talk) 18:01, 20 January 2010 (UTC)
← While O-ring erosion and evidence of blow-by on previous missions certainly indicated the problem that led to the STS-51-L loss, the effects on flight dynamics cannot be compared. Whereas the worse previous case, STS-51-C, had resulted in "unprecedented penetration of the primary O-Ring and heavily charred effects on the secondary O-Ring" in the center field joint of the right SRB, the aft field joint of the right SRB of STS-51-L, which was recovered from the debris, showed a 27 in. x 15 in. hole burned through the SRB casing. (photo) While wondering if the vehicle could had survived had the flames not been directed onto the ET could be regarded as idle curiosity, such a question would seem to influence future probabilistic risk assessment. That is, would any such burn through necessarily result in the loss of vehicle? 124.157.247.221 (talk) 23:20, 20 January 2010 (UTC)
What mean east and west speaking of the Antartic ?
Hello you nice Pythias. Please excuse my poor English, I'm French.
I already asked this question in the French equivalent of your reference desk called the Oracle but I got poor answers.
Introduction : very often, speaking of the melting of the antartic cap (I know it's a continent, but there's ice on and around the continent), specialists speak of the western part of the Antartic.
Question : This western makes a sense for me for Europe, Africa and every where else in the world except Artic and Antartic.
What does it mean for the Antartic ?
Thak you very much for your explainations. Joël DESHAIES - Rheims in France - --90.7.206.69 (talk) 14:24, 20 January 2010 (UTC)
We have West Antarctica. The French ref desk is called the Oracle? Over here we are explicitly not a crystal ball. I think that excludes all forms of divination. 81.131.36.119 (talk) 14:31, 20 January 2010 (UTC)
- More generically, an Oracle is one who gives wise answers to questions - possibly by consulting, not a crystal ball, but a relational database. ←Baseball Bugs What's up, Doc? carrots→ 02:27, 21 January 2010 (UTC)
- When you are exactly at the pole (either North or South), it's true to say that the words "East" and "West" don't mean anything. Once you get away from the pole, they have their usual meaning. The problem with the artctic/antarctic is that any place you happen to be standing is both east of one place and west of another. However, the antarctic is conveniently split in two by the 'Transantarctic Mountains' - and it has been agreed that the side of the continent that's to the west of the Weddel Sea is called "West Antarctica" and the other half "East Antarctica". This is a bit confusing when you are on the side of the continent nearest to the Ross Sea because West Antarctica is to the east of you and East Antarctica is to the west. But that's the price you pay for living in such a geographically confusing place! However, that division makes some sort of sense because we talk about the Earth as having a "Western Hemisphere" and an "Eastern Hemisphere" - and West Antarctica is in the Western Hemisphere with East Antarctica in the Eastern Hemisphere. Of course the names of the two hemispheres is also a bit weird if you happen to live on the International Date Line and the Eastern hemisphere is to your west and the Western Hemisphere to your east...but that's what you get when you live on a sphere. Topology is a harsh mistress! SteveBaker (talk) 14:37, 20 January 2010 (UTC)
- Thank you for for your good explainations, I think it's conclusive. Joël DESHAIES - Rheims in France ---90.7.206.69 (talk) 15:02, 20 January 2010 (UTC)
- Obligatory xkcd link --Tango (talk) 02:36, 21 January 2010 (UTC)
- That cartoon doesn't really work when you live in the UK...especially if you happen to be near Greenwich. SteveBaker (talk) 14:26, 21 January 2010 (UTC)
- Speaking as someone who lives in Greenwich, I used to live in the west but I moved a mile and half away, so now live in the east. Mikenorton (talk) 17:01, 21 January 2010 (UTC)
- Speaking of West Antarctica, it appears that Pine Island Bay, a major "tipping point" for the destabilization of the West Antarctic Ice Sheet has already passed its own tipping point for eventual collapse. ~AH1(TCU) 02:41, 23 January 2010 (UTC)
- Speaking as someone who lives in Greenwich, I used to live in the west but I moved a mile and half away, so now live in the east. Mikenorton (talk) 17:01, 21 January 2010 (UTC)
- That cartoon doesn't really work when you live in the UK...especially if you happen to be near Greenwich. SteveBaker (talk) 14:26, 21 January 2010 (UTC)
What kind of fish is this?
Thanks. --✶♏ݣ 15:17, 20 January 2010 (UTC)
- Chelmon rostratus, the Copperband butterflyfish. 124.157.247.221 (talk) 15:47, 20 January 2010 (UTC)
- Thank you! --✶♏ݣ 15:55, 20 January 2010 (UTC)
Do Solids burn
Does a solid burn or does it change state to a gas first ? Purple pete2000 (talk) 19:37, 20 January 2010 (UTC)
- Have a look at burning, especially the paragraph labelled 'solid fuel'. It says it does turn to gas. Richard Avery (talk) 19:42, 20 January 2010 (UTC)
- If you are thinking that the heat from the flame vaporizes the solid and then the gas burns - then definitely no. For a chunk of solid carbon (like maybe a charcoal briquette) turn into a carbon gas would require a temperature of 3642 °C - but carbon burns easily at around 700 °C when there is nowhere near enough heat to cause the carbon to vaporize. However, maybe there is some other weird thing happening right at the point of ignition. Fire is weird stuff. SteveBaker (talk) 23:52, 20 January 2010 (UTC)
- The answer is, of course, that it depends. Mostly, this depends on the solid fuel material, and the presence and quantity of oxidizer. Surprisingly, we have no article on flame dynamics, but here is Dynamics of Deflagrations and Reactive Systems: Flames (Progress in Astronautics and Aeronautics) ($104). Naturally I'm fascinated by this sort of thing. When designing my hybrid-fuel rocket [9], which used a liquid/gas-phase oxidizer and a solid-phase fuel, the ultimate question was to what extent can we control the thermodynamics of the phase transitions to optimize the specific impulse? I think the answer was, "50%," or something. The goal in our case was to keep the chamber temperature hot so that the fuel would gassify, and to keep the oxidizer flowing at the ideal rate to match the gassification rate of the fuel, and to do this without introducing oscillation. The trouble is, especially in the specifics of the particular chemicals we used, that the flow rate of the oxidizer is nonlinear (being choked at the inlet, and undergoing a phase change) - in fact, it spurts out in a combination of gas-phase and liquid vapor mist - each with different burn properties. Also, the fuel can burn in multiple modes - in solid form, in liquid form, in gaseous form (diffusion flame, roughly), and in "macrscopic chunks of fuel flying out the nozzle and igniting in flight." Anyway, the chamber can't start out hot - you have to heat it with flame! So the ignition must occur with a solid-phase fuel. To speed this along, we used a pyrotechnic igniter and blew hot fiery chunks of explosive which embedded into the walls of the fuel and ignited it in solid form right as we opened up the oxidizer flow. The process of switching flame modes from solid- to gassified- fuel is visible in some of the technical readouts and thrust curves, but it's hard to decouple from the effects of the oxidizer flow ramp-up. And if you're willing to supply a really powerful oxidizer, like fluorine gas, you can burn almost anything in solid form - even chicken. (Note that they don't even need an ignition source! Solid chicken will spontaneously ignite at room temperature in the presence of concentrated fluorine). So, the answer is, of course, it depends. Nimur (talk) 03:39, 21 January 2010 (UTC)
- I like how they give a purity of the chicken starting-material. DMacks (talk) 04:17, 21 January 2010 (UTC)
- The answer is, of course, that it depends. Mostly, this depends on the solid fuel material, and the presence and quantity of oxidizer. Surprisingly, we have no article on flame dynamics, but here is Dynamics of Deflagrations and Reactive Systems: Flames (Progress in Astronautics and Aeronautics) ($104). Naturally I'm fascinated by this sort of thing. When designing my hybrid-fuel rocket [9], which used a liquid/gas-phase oxidizer and a solid-phase fuel, the ultimate question was to what extent can we control the thermodynamics of the phase transitions to optimize the specific impulse? I think the answer was, "50%," or something. The goal in our case was to keep the chamber temperature hot so that the fuel would gassify, and to keep the oxidizer flowing at the ideal rate to match the gassification rate of the fuel, and to do this without introducing oscillation. The trouble is, especially in the specifics of the particular chemicals we used, that the flow rate of the oxidizer is nonlinear (being choked at the inlet, and undergoing a phase change) - in fact, it spurts out in a combination of gas-phase and liquid vapor mist - each with different burn properties. Also, the fuel can burn in multiple modes - in solid form, in liquid form, in gaseous form (diffusion flame, roughly), and in "macrscopic chunks of fuel flying out the nozzle and igniting in flight." Anyway, the chamber can't start out hot - you have to heat it with flame! So the ignition must occur with a solid-phase fuel. To speed this along, we used a pyrotechnic igniter and blew hot fiery chunks of explosive which embedded into the walls of the fuel and ignited it in solid form right as we opened up the oxidizer flow. The process of switching flame modes from solid- to gassified- fuel is visible in some of the technical readouts and thrust curves, but it's hard to decouple from the effects of the oxidizer flow ramp-up. And if you're willing to supply a really powerful oxidizer, like fluorine gas, you can burn almost anything in solid form - even chicken. (Note that they don't even need an ignition source! Solid chicken will spontaneously ignite at room temperature in the presence of concentrated fluorine). So, the answer is, of course, it depends. Nimur (talk) 03:39, 21 January 2010 (UTC)
- In a technical sense, no, because as these fellows have stated, a flame is basically ignited gas, so if you have ignition you always had gas as a precursor. That said, yes, solids do burn, if you don't worry about the transitional phase between solid and flame. Phosphorous being the classic example. It spontaneously ignites in air. Vranak (talk) 13:13, 23 January 2010 (UTC)
Latitudes and longitudes with the most land or the most water
I don't think there is a single latitude or longitude consisting either completely of land or completely of water. But which latitudes and longitudes come the closest? JIP | Talk 19:51, 20 January 2010 (UTC)
- Actually, while there are no longitudes consisting either completely of land or completely of water, there are such latitudes near the poles. Latitudes near the south pole consist entirely of land, and there might be some just to the north of them that consist entirely of water. Near the north pole it's mostly the same situation, depending whether you view the Arctic as land or not. Underneath all that ice is only water, no solid land, but because it's so cold there, the ice never melts, and thus no one actually gets to see that water underneath it. Other than the poles, which latitudes come the closest to being mostly land or mostly water? And what about the longitudes? JIP | Talk 19:54, 20 January 2010 (UTC)
- To find lines of latitude with only water (or land) take a conventionally-oriented sinusoidal projection of the earth's surface (as in File:Sinusoid-projection.jpg) and lay a ruler horizontally across it. Features running horizontally are scaled correctly in this projection, so just find the widest point of the map that's all water (or all land). Eyeballing, the longest line of latitude without land is just south of the tip of South America, around 55° south. The longest line that's all land (and, indeed, the only place where this occurs) is somewhere in Antarctica.
- For lines of longitude, you'd need to find a sinusoidal projection with the central meridian along the equator; I don't know where you'd get that. As JIP notes, all lines of longitude will pass through both the antarctic landmass and its surrounding oceans, so there are no completely wet or completely dry lines of longitude. TenOfAllTrades(talk) 20:19, 20 January 2010 (UTC)
- What about 89 degrees south, that is all land, but covered with a lot of ice. And 89 degrees north is all sea, but covered with a lot of ice again. 92.29.57.199 (talk) 21:37, 20 January 2010 (UTC)
- The International Date Line passes mainly through ocean, but it has to be diverted from longitude 180 degrees (W or E) to achieve this. Dbfirs 23:44, 20 January 2010 (UTC)
- A look at the map shows that the longitude with least land is right around 170W -- there are only about 5 degrees of latitude of land there. The longitude with the most land is near 25E, with close to 120 degrees of latitude of land. Looie496 (talk) 00:25, 21 January 2010 (UTC)
- The International Date Line passes mainly through ocean, but it has to be diverted from longitude 180 degrees (W or E) to achieve this. Dbfirs 23:44, 20 January 2010 (UTC)
Virginity
I'm curious, does anyone have statistics on the distribution of ages at which people experience their first sexual intercourse? For example, (pulling numbers out of the air) perhaps 10% of people lose their virginity at age 15, 15% at age 16, 15% at age 17, and so on... I sometimes see claims about the average age at which people give up their virginity, but such reports almost never present much real data. I'm wondering if people know of any detailed data sets. Dragons flight (talk) 21:28, 20 January 2010 (UTC)
- Here you go. I found this by just googling "first sexual experience statistics" and hunting around a bit there. (Note that this is a U.S.-only dataset. I'm not sure global statistics are as available, but you can probably find similar things for all Western countries.) --Mr.98 (talk) 21:40, 20 January 2010 (UTC)
- There was a similar question to this recently. 92.29.57.199 (talk) 23:32, 20 January 2010 (UTC)
- There will also be issues gathering this data as it will be self reported data, and such data is often not fully accurate. Googlemeister (talk) 14:22, 21 January 2010 (UTC)
- I'm puzzled by "Average age of first intercourse by gender" in the Kinsey report. It seems that at every age grouping a higher percentage of girls have had sex than boys yet, on average, boys lose their virginity earlier. Is that consistent?--Frumpo (talk) 22:09, 21 January 2010 (UTC)
- I don't think so. However, there is one age group (20-21) at which the male percentage exceeds the female one, and data aren't given for ages over 24, so a strategically placed wiggle in the data at either or both of these levels could cause the effect. For example, perhaps most of the remaining 11% of males all lose their virginity before they're thirty, while more of the 8% of females do so at older ages. If this is true, though, that would be almost a more interesting fact about the distributions than the ones presented! It would be very nice to see a relative distribution (in the sense of Handcock and Morris). Neurotip (talk) 10:44, 22 January 2010 (UTC)
- I'm puzzled by "Average age of first intercourse by gender" in the Kinsey report. It seems that at every age grouping a higher percentage of girls have had sex than boys yet, on average, boys lose their virginity earlier. Is that consistent?--Frumpo (talk) 22:09, 21 January 2010 (UTC)
January 21
Plants
What evolutionary advantage is there for plants to taste good? It seems like this would make them more likely to be eaten by animals, and thus less favored by natural selection, but obviously this isn't the case. --75.15.162.220 (talk) 00:52, 21 January 2010 (UTC)
- Being eaten can actually be an advantage. The biggest problem most plants face is to spread their seeds to distant locations -- setting things up so that animals scatter the seeds in the process of eating part of the plant is one way of accomplishing that. Looie496 (talk) 00:56, 21 January 2010 (UTC)
- I think you're thinking of this situation backwards. Plants produce sugars which they use for fuel, reproduction, etc. For animals, it is an evolutionary advantage to know what is useful to eat and what is not. Extrapolate a few thousand (million?) generations and things that you should eat taste good, things you shouldn't (or needn't) tend to taste bad. As for why things that are "bad for you" taste so good - what makes them taste good is actually good for you, in smaller portions. We simply eat too much and become rubenesque. (well, not ME! ;-)) 218.25.32.210 (talk) 00:59, 21 January 2010 (UTC)
- That makes sense for why unhealthy foods can taste good, but it doesn't explain why, to most people, at least, healthy foods taste bad. --75.15.162.220 (talk) 01:05, 21 January 2010 (UTC)
- Your "unhealthy" foods aren't really unhealthy. This is an important realization. They're just (artificially) too rich in nutrients / we eat too much of them. 218.25.32.210 (talk) 01:58, 21 January 2010 (UTC)
- But why do most people think that "healthy" foods (vegetables, soy, etc.) taste bad, despite their obvious health benefits? --71.153.45.189 (talk) 02:03, 21 January 2010 (UTC)
- Cultural norms? There are approximately 1.3 billion people in China who would likely disagree with your assertion that soy (or, by implication, tofu) tastes bad. Likewise, in my experience here most Chinese cannot handle the tremendous sweetness of an American confection like a Peep. This is not an evolutionary difference, as Mr. 98 alludes to below. 218.25.32.210 (talk) 03:04, 21 January 2010 (UTC)
- Americans are used to sugar, so tofu looks and tastes like library paste to us. But I suppose if one were raised on tofu, it might become palatable. Although that hasn't worked for lutefisk, which your typical Norwegian regards as unhealthful AND bad-tasting, yet they eat it anyway. ←Baseball Bugs What's up, Doc? carrots→ 03:20, 21 January 2010 (UTC)
- I don't think that people think healthy foods taste bad. They just taste worse than other modern alternatives like medium rare steaks and chocolate bars - they do taste better than poison, though. Zain Ebrahim (talk) 08:20, 21 January 2010 (UTC)
- I think steaks and chocolate bars are sickening - fruit and vegetables are much nicer. Americans have been conditioned to like fat, sugar, and salt. But it is possible to loose your taste for these too. 92.24.85.238 (talk) 21:04, 21 January 2010 (UTC)
- Isn't antifreeze supposed to taste pretty good? Googlemeister (talk) 14:20, 21 January 2010 (UTC)
- (edit conflict) Indeed. As an example, the sweetness of corn has risen dramatically, leaving the original stuff tasteless at best by our standards. ~ Amory (u • t • c) 14:26, 21 January 2010 (UTC)
- Cultural norms? There are approximately 1.3 billion people in China who would likely disagree with your assertion that soy (or, by implication, tofu) tastes bad. Likewise, in my experience here most Chinese cannot handle the tremendous sweetness of an American confection like a Peep. This is not an evolutionary difference, as Mr. 98 alludes to below. 218.25.32.210 (talk) 03:04, 21 January 2010 (UTC)
- But why do most people think that "healthy" foods (vegetables, soy, etc.) taste bad, despite their obvious health benefits? --71.153.45.189 (talk) 02:03, 21 January 2010 (UTC)
- Your "unhealthy" foods aren't really unhealthy. This is an important realization. They're just (artificially) too rich in nutrients / we eat too much of them. 218.25.32.210 (talk) 01:58, 21 January 2010 (UTC)
- That makes sense for why unhealthy foods can taste good, but it doesn't explain why, to most people, at least, healthy foods taste bad. --75.15.162.220 (talk) 01:05, 21 January 2010 (UTC)
- An issue in all of these discussions about natural selection is that 1. humans and their culture have changed a huge amount since we initially evolved food preferences, and 2. our plants have changed quite a bit as well. The vegetables that we eat in modern industrial countries bear little resemblance in many cases to their original forms. An evolutionary model can get you some places, but it can't get you all of them. Culture, history, etc. need to fill the gap. --Mr.98 (talk) 02:16, 21 January 2010 (UTC)
- It's important to distinguish between the parts of a plant where it is advantageous for the plant species that they are eaten (the fruit) and those parts where it is disadvantageous (the leaves, etc.). Plants may evolve to have pleasant tasting fruit and unpleasant tasting leaves. Unfortunately for them, animals are also evolving and those individuals that find the leaves palatable may do better than those that don't. So plants might evolve bitter-tasting leaves but animals evolve which enjoy the bitter taste. It's a war out there.--Frumpo (talk) 11:40, 21 January 2010 (UTC)
- And animals like humans happily subvert the intentions of the plant, anyway, by learning how to cook out the bad stuff, amplify the good stuff, and even breed out the seeds. --Mr.98 (talk) 13:24, 21 January 2010 (UTC)
- The key point here is that the vital materials of one organism will often be compatible with another. So being delicious and being healthy are more or less overlapping. Vranak (talk) 22:11, 21 January 2010 (UTC)
Numbers
This may sound foolish, but are there any numbers that don't exist, are unquantifiable, or have yet to be defined? I'm not thinking of infinity but of strange 'dark matter' numbers that we have not yet found —Preceding unsigned comment added by 86.180.35.161 (talk) 01:36, 21 January 2010 (UTC)
- There's nothing you can do that can't be done. There's nothing you can sing that can't be sung. There are no numbers that don't exist. --Trovatore (talk) 01:45, 21 January 2010 (UTC)
- Numbers are a human invention. There are the "real" numbers and the so-called "imaginary" [a.k.a. "complex"] numbers, i.e. numbers that are a function of the square root of -1. But even the "imaginary" numbers are as "real" as any other numbers in the conceptual sense. ←Baseball Bugs What's up, Doc? carrots→ 01:48, 21 January 2010 (UTC)
- How many humans invented numbers? --Trovatore (talk) 02:18, 21 January 2010 (UTC)
- A number of them. ←Baseball Bugs What's up, Doc? carrots→ 03:17, 21 January 2010 (UTC)
- Numbers are by no means a human invention! They transcend the world but they also permeate it. Numbers essentially speak of relationships between things. Even without human intellect, things exist, and they are in relationships with each other, even if no one is around to take notes. Vranak (talk) 19:55, 21 January 2010 (UTC)
- How many humans invented numbers? --Trovatore (talk) 02:18, 21 January 2010 (UTC)
- Numbers are a human invention. There are the "real" numbers and the so-called "imaginary" [a.k.a. "complex"] numbers, i.e. numbers that are a function of the square root of -1. But even the "imaginary" numbers are as "real" as any other numbers in the conceptual sense. ←Baseball Bugs What's up, Doc? carrots→ 01:48, 21 January 2010 (UTC)
well not so long ago we didn't have negative numbers, fractions and exponential numbers. We do now, so therefore are there numbers we have missed in a long tradition of missing numbers? —Preceding unsigned comment added by 86.180.35.161 (talk) 02:09, 21 January 2010 (UTC)
- And just how would we know? ←Baseball Bugs What's up, Doc? carrots→ 02:17, 21 January 2010 (UTC)
- Ah, that's a different question. A great many structures are known whose elements are occasionally called numbers by somebody. They don't really have much in common. There are certainly interesting structures yet to be discovered; whether you call their elements "numbers" or not is fairly arbitrary, even if the structures themselves are not. --Trovatore (talk) 02:18, 21 January 2010 (UTC)
This question may receive more insightful answers on the math desk. I think that in earlier centuries, concepts about numbers were less well developed - as mentioned above, critical concepts about number theory had not been developed yet, so there was no human understanding about negative numbers, complex numbers, and so forth, until those ideas were first informally and later formally studied. Modern mathematical number theory is so well developed, so sufficiently abstract, that it would be very hard to conceive of a new type of number that both fits the definition of a number, and also fails to meet the criteria for inclusion in one of the existing categories. This is especially true of the very abstract set theory representations - for example, see Set-theoretic definition of natural numbers for an introduction to this methodology. If you, or anybody, could conceive a new type of number, it would probably garner a lot of attention from theoretical mathematicians. Finally, a more trivial answer to your question is that there are certainly large numbers who have never been represented in any form, ever before, by human or machine. All you have to do to generate one such number is to take the largest number which has ever been previously expressed, and add 1 to it (or multiply it by 357, and so forth). In that sense, any member of that infinite, uncountable set of specific numbers that have never been "written down" somewhere has never been discovered by humans. And this is just the integers! I won't even begin to discuss the subtlety of the real number spectrum! There's an uncountably infinite set of numbers between every two numbers that can be expressed! And so, there is an uncountably infinite number of sets, each of which contains an uncountably infinite number of numbers - have a read about degrees of infinity at the Cardinality article for more. But the significance of "discovering" a never-before-seen member of this set is moot - it doesn't surprise any mathematician (or anyone, really), because it's just another number which satisfies the properties we expect it to satisfy. Nimur (talk) 03:11, 21 January 2010 (UTC)
- This would be a good time to reference the philosophy of mathematics article. Are numbers (or any mathematical concept) "discovered", or are they "invented"? I'm in the "invented" camp (I identify with the Formalists), but it's not a trivial question. Buddy431 (talk) 03:23, 21 January 2010 (UTC)
- I can't resist droning on a bit more - this time from the perspective of a computer scientist, rather than a mathematician. Every number can be thought of as the uniquely defined hash of a unique algorithm (even if we do not actually know the hash function used to generate it). And, there are plenty of algorithms that we hope to design, but do not yet know if it is theoretically possible to design. In that sense, if you could concoct a method to generate the numbers that represent the algorithms we hope to build, you might have something great - but this is more of a matter of designing a new kind of function that generates numbers. Strictly speaking, it is not a new class of numbers. Nonetheless, it's an unsolved, number-related problem at the frontier of human knowledge. Nimur (talk) 03:26, 21 January 2010 (UTC)
- This is absolutely false, at least in the universe of the standard real numbers. Every computable number comes from an algorithm, but there are lots of others that don't. Most real numbers are not computable. Staecker (talk) 05:30, 21 January 2010 (UTC)
- I can't resist droning on a bit more - this time from the perspective of a computer scientist, rather than a mathematician. Every number can be thought of as the uniquely defined hash of a unique algorithm (even if we do not actually know the hash function used to generate it). And, there are plenty of algorithms that we hope to design, but do not yet know if it is theoretically possible to design. In that sense, if you could concoct a method to generate the numbers that represent the algorithms we hope to build, you might have something great - but this is more of a matter of designing a new kind of function that generates numbers. Strictly speaking, it is not a new class of numbers. Nonetheless, it's an unsolved, number-related problem at the frontier of human knowledge. Nimur (talk) 03:26, 21 January 2010 (UTC)
- Calling composite things like
imaginarycomplex numbers "numbers" is a weird kink that mathematicians have gotten into. I can't think why - it's an entirely illogical move. Animaginarycomplex number is just a 2D vector - the operations we do onimaginarycomplex numbers are just regular vector operators - why make such a big deal over it? Basically, you can do arithmetic on things like the square root of a negative number if you treat the intermediate values as vectors. Big deal - that doesn't demand an entire new class of number.
- A more reasonable view of what a number is would be a position on a 1D line that stretches from negative infinity to positive infinity. Every "number" is represented by a point along that line. Integers, rational and irrational numbers are just different points along that line. Taking that simple definition - the OP's question is answerable:
- are there any numbers that don't exist ? - No, they are all on the line somewhere. There are no mysterious gaps.
- are unquantifiable ? - A number is a quantity. Can a quantity be unquantifiable? No.
- or have yet to be defined ? - What do you mean by "defined"? A number is it's own definition...but let's come back to this one in a moment.
- strange 'dark matter' numbers that we have not yet found ? - Can something not be 'found' when we know that (by definition) it lies somewhere on that line?
- But you obviously seek mystery and excitement amongst numbers - and perhaps we can find some stuff like that:
- There are numbers along that line that humans have never used - write down a number with 500 random digits and the odds are very good that nobody else will ever have written down that number - or used it for anything and that it doesn't represent anything at all in our universe - it's a brand new unused number! But it's been there on the number line for as long as we've had the number line. It's no surprise that it's there or that it exists. It's not really a surprise or particularly novel in any way.
- Arguably the oddest kinds of numbers are the irrational numbers - stuff like pi and 'e'. They represent a point on the line - but pointing at that point is kinda tricky because they require an infinite number of digits. If our questioner wants an oddity, then coming up with a 'new' irrational number would be the nearest thing to that. Sadly, it's very easy to make a new irrational. Just add pretty much any number to pi and you have a brand new irrational number all of your own. But pi has a 'definition' (it's the ratio between the diameter and the circumpherence of a circle). There are other irrational numbers that we have not yet "discovered" - and numbers like Catalan's constant that we are (so far) unable to determine whether they are irrational or not.
- Humans haven't always known this though - plenty of people in the past didn't 'believe' in negative numbers or did not accept the concept of the number zero. Adding those concepts to our understanding is a relatively new thing. Understanding the irrational numbers is another thing that is relative new in our history. Perhaps there are other odd-ball classes of numbers out there that we haven't thought of yet - that would create the mystique that I think you're looking for.
- For the notational problems associated with writing down the value of really, REALLY large numbers, see some of the last few sections of our Large numbers article.
- You might also want to read our 0.999... article.
- SteveBaker (talk) 04:12, 21 January 2010 (UTC)
- I agree with everything here, except your description of imaginary numbers as "2d vectors". I can't think of how you mean that. Are you certain you're not thinking of Complex numbers? APL (talk) 04:25, 21 January 2010 (UTC)
- Yeah - sorry - I did mean "complex" (I've fixed my response). But in my defense - until you put the imaginary part (without the 'i' - or the 'j' if you're an electrical engineer) into one element of a 2D vector and call it a "complex number" - it's really totally useless. All discussions of the importance and value of imaginary numbers are really talking about the importance and value of complex numbers - and all of those discussions immediately jump to a little 2D image that shows the complex number is really being treated as a vector. So all of this talk about imaginary/complex numbers could simply be boiled down to a rather simple application of vectors. SteveBaker (talk) 14:09, 21 January 2010 (UTC)
- (ec) I'm not sure why we should insist that our fields be ordered to consider their elements to be "numbers". The complex numbers behave (in many ways) very much like the more traditional "1D line that stretches from negative infinity to positive infinity". I, for one, appreciate the fact that I can find three solutions to the equation x3-1=0, and I don't really mind that I can't decide which solution is biggest. To give another (weirder) example of objects known as "numbers" that Steve won't like, I refer you to the Quaternions (they're like 4D vectors!). And since no one has yet linked to the plain old number article, I figure I can do that now. It gives some insight into when (and why) different types of numbers have been invented (that's right, invented) if anyone's curious. Buddy431 (talk) 05:18, 21 January 2010 (UTC)
- But that same comment applies with just as much validity to (for example) 3D vectors as it does to 2D vectors and complex numbers - so is the position of an object in 3D space just a "number"? What about a description of the shape of a polynomial curve with a bunch of coefficients? Is that a "number" too? Where do you stop? Is an elephant just a number? What you do by getting excited about generalizing the concept of a number beyond a simple position on a 1D line is to turn the term into a worthless pile of mush that could be applied to anything. That's an irresponsible thing to do. If you need a word for numbers and vectors and matrices and other things like that - then invent a new word - don't corrupt the meaning of the original word. SteveBaker (talk) 14:09, 21 January 2010 (UTC)
- No, my comment does not apply with "just as much validity" to 3D vectors. The 2D complex number plane truly is different from higher dimension fields, as Staecker elequently explains below. Hamilton tried to develop a 3D version of the complex plane, but failed. He eventually realized that he could get close with a 4D quaternion system, though even there you lose commutativity. Buddy431 (talk) 01:00, 22 January 2010 (UTC)
- Yeah - all of the lower orders of vector have different properties and there are things like cross products that don't have meaning other than in specific numbers of dimensions. You're talking like I don't know what a quaternion is - I've been programming computer graphics applications and engines using 4D vectors that I sometimes use for representing rotations and such ("quaternions") for 20 years. I happen to know that mathematicians want to complicate the issue with yet another special-purpose name and call them "quaternions" instead of "4D vectors" - but for me the quaternions are just another set of operations on 4D vectors. But for me, there isn't anything special about 'quaternions'. I define operators that take a 4D vector that represents the equation of a 3D plane (Ax + By + Cz == D) and perform operations on that plane - or to store a space-time coordinate in an animation - I use the same sets of operations to perform rotations in 3D space...I don't know (or care) whether mathematicians want to give this yet another tediously painful to learn name - it's just another member of the general algorithmic toolkit that works with 4D vectors - and quaternion multiplication, rotation, spherical interpolation are others. But splitting apart that set of operators that work on "complex numbers" from those that operate on "2D vectors" is a collosal waste of my time and a horribly complicating factor that would impede me from using these tools. So - I have 2D vectors and some really simple rules for how to use 2D vectors to represent things like the square root of a negative number using the same vector toolkit I use to plot graphs and do plane geometry. Imaginary (or maybe it's complex) numbers are just part of the bizarre, incomprehensible pile of vocabulary that mathematicians use to obfuscate their work form us 'mere mortals' who use this stuff every day. Shed that stuff and vector/matrix math is easy. SteveBaker (talk) 03:53, 22 January 2010 (UTC)
- Steve, I'm not sure if you've missed the point here, or if you're trying to slough over it. No doubt for your specific set of graphical applications, you can just remember these transformations on vectors and that's all you need. But what you're missing is the regularities among them that are much more easily conceptualized in complex-variable language. It's not about obfuscation, just the opposite; it's a clarifying conceptual step. Not so much when you're doing one rotation/scaling at a time, no. But for lots of other things.
- The first nontrivial application is from algebra; just knowing that every polynomial can be factored all the way down to linear factors over the complex numbers. That's already very useful, and how you'd phrase it in terms of rotations and scalings I have no idea.
- But things don't really get going until you hit analytic functions, which are simply functions that have a derivative in terms of the complex numbers. Over the reals, having a derivative is no big deal. Over the complex numbers, it's very restrictive indeed, and tells you a lot about the function. This information can be leveraged in all sorts of surprising ways (this is how the prime number theorem is most easily proved, for example; I imagine you care about that, given that it affects your banking security).
- I remember a student asking me once, in a College Algebra class, why she should learn about the complex numbers. Now at some level it wasn't an unreasonable question, because frankly the whole class is useless — it's a grab-bag of algorithms that will be memorized by the students long enough to pass the final, but never understood at a level that could do the student any real good. Just the same I was unembarrassed to tell her that she should learn about complex numbers simply because they're really really cool.
- (What I may have left unsaid, don't remember for sure, was that unfortunately she would never get to see that coolness — in College Algebra all you see is that every polynomial can be factored down to linear factors, which is not something she'd appreciate beyond the mere formality of it, and the chance of a College Algebra student ever taking a class where analytic functions show up is negligible; by definition, College Algebra students hate mathematics, and College Algebra itself is certainly not designed to change their minds on that point. Pity that. Still, my answer was true and honorable.) --Trovatore (talk) 08:22, 22 January 2010 (UTC)
- Yeah - all of the lower orders of vector have different properties and there are things like cross products that don't have meaning other than in specific numbers of dimensions. You're talking like I don't know what a quaternion is - I've been programming computer graphics applications and engines using 4D vectors that I sometimes use for representing rotations and such ("quaternions") for 20 years. I happen to know that mathematicians want to complicate the issue with yet another special-purpose name and call them "quaternions" instead of "4D vectors" - but for me the quaternions are just another set of operations on 4D vectors. But for me, there isn't anything special about 'quaternions'. I define operators that take a 4D vector that represents the equation of a 3D plane (Ax + By + Cz == D) and perform operations on that plane - or to store a space-time coordinate in an animation - I use the same sets of operations to perform rotations in 3D space...I don't know (or care) whether mathematicians want to give this yet another tediously painful to learn name - it's just another member of the general algorithmic toolkit that works with 4D vectors - and quaternion multiplication, rotation, spherical interpolation are others. But splitting apart that set of operators that work on "complex numbers" from those that operate on "2D vectors" is a collosal waste of my time and a horribly complicating factor that would impede me from using these tools. So - I have 2D vectors and some really simple rules for how to use 2D vectors to represent things like the square root of a negative number using the same vector toolkit I use to plot graphs and do plane geometry. Imaginary (or maybe it's complex) numbers are just part of the bizarre, incomprehensible pile of vocabulary that mathematicians use to obfuscate their work form us 'mere mortals' who use this stuff every day. Shed that stuff and vector/matrix math is easy. SteveBaker (talk) 03:53, 22 January 2010 (UTC)
- No, my comment does not apply with "just as much validity" to 3D vectors. The 2D complex number plane truly is different from higher dimension fields, as Staecker elequently explains below. Hamilton tried to develop a 3D version of the complex plane, but failed. He eventually realized that he could get close with a 4D quaternion system, though even there you lose commutativity. Buddy431 (talk) 01:00, 22 January 2010 (UTC)
- But that same comment applies with just as much validity to (for example) 3D vectors as it does to 2D vectors and complex numbers - so is the position of an object in 3D space just a "number"? What about a description of the shape of a polynomial curve with a bunch of coefficients? Is that a "number" too? Where do you stop? Is an elephant just a number? What you do by getting excited about generalizing the concept of a number beyond a simple position on a 1D line is to turn the term into a worthless pile of mush that could be applied to anything. That's an irresponsible thing to do. If you need a word for numbers and vectors and matrices and other things like that - then invent a new word - don't corrupt the meaning of the original word. SteveBaker (talk) 14:09, 21 January 2010 (UTC)
- I agree with everything here, except your description of imaginary numbers as "2d vectors". I can't think of how you mean that. Are you certain you're not thinking of Complex numbers? APL (talk) 04:25, 21 January 2010 (UTC)
- (ec) FYI Steve- what makes complex numbers much much more interesting than vectors is that you can multiply and divide them. See Complex number. This fact allows you to do lots and lots of things that you can't typically do with vectors- such as define the complex derivative, which requires division. This is a very big deal, and not just in pure mathematics. Engineers and scientists have been using basic results of complex analysis for years. You might wonder whether similar multiplication operations could be defined on higher-dimensional vector spaces, and the answer is basically no. The only dimensions in which a multiplication operation can be defined which is commutative, associative, and distributive are: 1 (the real numbers) and 2 (the complex numbers). (There's also the quaternions in dimension 4 if you discard commutativity.) So you could say they're "just 2D vectors" if you like, but all the great things you can do with complex numbers are provably impossible in any higher dimensions. This is the Frobenius theorem. Staecker (talk) 05:27, 21 January 2010 (UTC)
- But those operators that you call 'multiply' and 'divide' on complex numbers are also useful general purpose operations on 2D vectors - I use those operators in computer graphics all the time - heck they are even in the standard vector software library I wrote. If history had come out a little differently we could well have not needed to think about complex numbers at all - and just talked about 2D vectors being a handy way to work around the problem of doing math with square roots of negative numbers. Ditto quaternions (just a bunch of handy operations on 4D vectors). Giving these things funny names just obscures the commonality between them and other branches of math and makes the practical application of math much more complicated and jargon-laden than it needs to be. SteveBaker (talk) 14:09, 21 January 2010 (UTC)
- Certainly if all you care about is vectors, then you'll want to emphasise the "commonality" between complex numbers and 2D vectors. But because of the multiplication and division operations (and some other things), you can do calculus with complex numbers. You can do derivatives and integrals and all the other stuff that you do in real number calculus. If calculus is what you care about, then you really should call them numbers. To insist on calling them vectors all the time "obscures the commonality" between them and numbers. Complex analysis is really much much more than some "handy operations" on 2D vectors. If you don't believe me try to formulate the Cauchy Riemann equations or Cauchy integral formula in terms of 2D vectors. You could do it, but it would be unbelievably tedious and very unnatural. And these are two of the most basic results in all of complex analysis. Take any more sophisticated recent theorems and it's absurd to think of them as vectors.
- All that said, if all you care about in math is vectors (if, say, you're doing computer graphics), then you won't care much about fancy complex analysis, and "they're just 2D vectors" is probably OK for you. But it's not the whole story. Staecker (talk) 15:50, 21 January 2010 (UTC)
- But those operators that you call 'multiply' and 'divide' on complex numbers are also useful general purpose operations on 2D vectors - I use those operators in computer graphics all the time - heck they are even in the standard vector software library I wrote. If history had come out a little differently we could well have not needed to think about complex numbers at all - and just talked about 2D vectors being a handy way to work around the problem of doing math with square roots of negative numbers. Ditto quaternions (just a bunch of handy operations on 4D vectors). Giving these things funny names just obscures the commonality between them and other branches of math and makes the practical application of math much more complicated and jargon-laden than it needs to be. SteveBaker (talk) 14:09, 21 January 2010 (UTC)
- There is also the octonions in dimension 8 if you discard both commutativity and associativity. Dauto (talk) 05:39, 21 January 2010 (UTC)
- Which we also have an article for, if anyone is looking for some light bedtime reading. Would it have been acceptable to go into Dauto's response and just link to the article there, or is that like the internet equivilent of telling a woman that she looks fat? Buddy431 (talk) 05:48, 21 January 2010 (UTC)
- Please don't do that. It's considered exceedingly bad etiquette to edit other people's posts - even to insert a benign-seeming link. The problem being that (in general) the original author might have intended a different meaning or twist on a word than you forced upon him by turning it into a link and thereby giving it a definite meaning. If I say "Man cannot live by bread alone" and you link Man then you've changed my meaning from "People in general" to "Male human", and if you link bread you've changed the meaning from "mundane earthly things" to "food made by baking flour and water with yeast" - which is a quite different statement than the one I intended. That probably wouldn't be an issue with a fairly obscure word like 'octonion' - but this is a point of principle. So, no - never edit other people's posts, except perhaps in extreme circumstances to fix some piece of wiki-formatting that's disrupting the remainder of the page such as an unbalanced <small> tag. You did the right thing. SteveBaker (talk) 14:18, 21 January 2010 (UTC)
- Which we also have an article for, if anyone is looking for some light bedtime reading. Would it have been acceptable to go into Dauto's response and just link to the article there, or is that like the internet equivilent of telling a woman that she looks fat? Buddy431 (talk) 05:48, 21 January 2010 (UTC)
- (ec) FYI Steve- what makes complex numbers much much more interesting than vectors is that you can multiply and divide them. See Complex number. This fact allows you to do lots and lots of things that you can't typically do with vectors- such as define the complex derivative, which requires division. This is a very big deal, and not just in pure mathematics. Engineers and scientists have been using basic results of complex analysis for years. You might wonder whether similar multiplication operations could be defined on higher-dimensional vector spaces, and the answer is basically no. The only dimensions in which a multiplication operation can be defined which is commutative, associative, and distributive are: 1 (the real numbers) and 2 (the complex numbers). (There's also the quaternions in dimension 4 if you discard commutativity.) So you could say they're "just 2D vectors" if you like, but all the great things you can do with complex numbers are provably impossible in any higher dimensions. This is the Frobenius theorem. Staecker (talk) 05:27, 21 January 2010 (UTC)
Much of the above answers don't seem very mathematically informed. The original poster may be interested in computable numbers. A number is "computable" if there is an algorithm (computer program or similar strictly definable procedure) which can compute its digits. Not all numbers have this property. In fact, it can be mathematically proven that "almost all" real numbers are not computable. There's another interesting notion: definable number. A "definable" number is one which can be defined unambiguously by a simple logical sentence in set theory. Again, it can be proven that almost all real numbers are not definable.
Every number you've ever heard of (unless you're an expert in this sort of thing) is both computable and definable, even though it can be proven that the vast majority of numbers have neither of these properties. Both of these concepts may provide examples of what you are looking for if you want an "unquantifiable" or "dark matter" style number. Almost all of them are like that. Staecker (talk) 05:07, 21 January 2010 (UTC)
- I don't think it's a matter of being "mathematically informed". The issue is that the original post can be interpreted by a mathematician, a physicist, a philosopher, a computer scientist, a linguist, etc. Each community contains a sort of different perspective on the interpretation of the question. Pretending that the pure theoretical mathematic approach is the only valid approach is ... well, what a theoretical mathematician would do. But the concept of number is so broad, so vague, and is used by so many different technical and research communities, that it is silly to pretend that mathematicians have a monopoly on its interpretation. Nimur (talk) 16:08, 21 January 2010 (UTC)
- When "technical and research communities" use the concept of numbers, they are all using the same concept. This is because they all use standard mathematics. Computer scientists use the real numbers, not their own version of the real numbers. Same goes for physicists. So when a computer scientist says that every real number corresponds to an algorithm, he is wrong. If you want to talk about the real numbers, then "the only valid approach" is to talk about the real numbers. Staecker (talk) 18:34, 21 January 2010 (UTC)
- Actually, no, computer scientists (except for those on the seriously hard-core theory side) don't use the real numbers at all. The real numbers are about geometry, topology, continuity; none of those concepts applies directly to computers, which have finite memories and therefore can represent only quantities with finite information.
- The "real numbers" used in real-world computers are actually a restricted set of dyadic rationals.
- Think of a real number as an infinite amount of information, all wrapped up in one neat little package. That has no direct application to CS (though, certainly, working with the reals conceptually is often more convenient and more powerful than working with discrete structures, and the outcome of that reasoning can be relevant to computers). --Trovatore (talk) 19:46, 21 January 2010 (UTC)
- (Ah, on re-reading, it looks as though I may have misinterpreted you somewhat -- apologies.) --Trovatore (talk) 19:49, 21 January 2010 (UTC)
- I think the key thing is that computer scientists use a subset of the real numbers. They don't use any numbers than aren't real numbers (or complex numbers - that's only a degree 2 extension, so doesn't make much difference). --Tango (talk) 19:51, 21 January 2010 (UTC)
- Well, actually, I don't really agree with that. The rational numbers are not real numbers; the real numbers are sui generis. The reals realize the intuition of a continuous line; that has little to do with the algebraic motivation behind the rationals.
- Of course the rationals are naturally embedded in the reals. However the IEEE float and double numbers (or more precisely the operations on them) do not respect that embedding, at least not exactly. So really the CS numbers are not even rationals; they're something a little different. --Trovatore (talk) 20:00, 21 January 2010 (UTC)
- At the risk of sounding very brusque - yes. IEEE-754 is the formal way that engineers say "screw off" to pompous mathematical types. We have our own definitions of numbers, operations on numbers, and consistent set of rules for working with them. We followed the theory as far as it was useful to do so, and we don't care about the subtlety any more than that. Now, we have to get back to programming our robots, rockets, and electric guitars - the math guys can complain all they want, but if they expect to do so using an internet-enabled computer, they are implicitly acknowledging that our system works. Nimur (talk) 00:47, 22 January 2010 (UTC)
- Hmm? Who's complaining? That chip on your shoulder's pretty big, doesn't it hurt your back? --Trovatore (talk) 03:00, 22 January 2010 (UTC)
- I think Staecker was complaining. As far as the chip on my shoulder hurting my back - you bet - my chip's huge...! : ) Nimur (talk) 03:46, 22 January 2010 (UTC)
- I'm not sure if I was complaining.. But of course I know that computers internally don't use real numbers, they use some type of rationals. I was trying to respond to above posts which suggest that computer scientists (not computers) don't believe in the real numbers, and that rational or computable numbers are all that exist. This would be like an grocery checkout clerk saying that the number $10.054 doesn't exist, because there's no such thing as .4 cents. But I think this has gone on long enough now... Staecker (talk) 13:19, 22 January 2010 (UTC)
- I think Staecker was complaining. As far as the chip on my shoulder hurting my back - you bet - my chip's huge...! : ) Nimur (talk) 03:46, 22 January 2010 (UTC)
- Hmm? Who's complaining? That chip on your shoulder's pretty big, doesn't it hurt your back? --Trovatore (talk) 03:00, 22 January 2010 (UTC)
- At the risk of sounding very brusque - yes. IEEE-754 is the formal way that engineers say "screw off" to pompous mathematical types. We have our own definitions of numbers, operations on numbers, and consistent set of rules for working with them. We followed the theory as far as it was useful to do so, and we don't care about the subtlety any more than that. Now, we have to get back to programming our robots, rockets, and electric guitars - the math guys can complain all they want, but if they expect to do so using an internet-enabled computer, they are implicitly acknowledging that our system works. Nimur (talk) 00:47, 22 January 2010 (UTC)
- I think the key thing is that computer scientists use a subset of the real numbers. They don't use any numbers than aren't real numbers (or complex numbers - that's only a degree 2 extension, so doesn't make much difference). --Tango (talk) 19:51, 21 January 2010 (UTC)
- (Ah, on re-reading, it looks as though I may have misinterpreted you somewhat -- apologies.) --Trovatore (talk) 19:49, 21 January 2010 (UTC)
- When "technical and research communities" use the concept of numbers, they are all using the same concept. This is because they all use standard mathematics. Computer scientists use the real numbers, not their own version of the real numbers. Same goes for physicists. So when a computer scientist says that every real number corresponds to an algorithm, he is wrong. If you want to talk about the real numbers, then "the only valid approach" is to talk about the real numbers. Staecker (talk) 18:34, 21 January 2010 (UTC)
- I don't think it's a matter of being "mathematically informed". The issue is that the original post can be interpreted by a mathematician, a physicist, a philosopher, a computer scientist, a linguist, etc. Each community contains a sort of different perspective on the interpretation of the question. Pretending that the pure theoretical mathematic approach is the only valid approach is ... well, what a theoretical mathematician would do. But the concept of number is so broad, so vague, and is used by so many different technical and research communities, that it is silly to pretend that mathematicians have a monopoly on its interpretation. Nimur (talk) 16:08, 21 January 2010 (UTC)
- ... and if you really want to stretch your imagination with numbers that are definitely on the dark side, try our articles on Hyperreal number, surreal number and superreal number. Dbfirs 09:02, 21 January 2010 (UTC)
- Mathematicians invent new types of numbers by playing a game called "what if ?". "What if there were a number whose square was -1 ?" - okay, let's call it i. What if we add i to itself ? What if we add 1 to i ? What if we multiply i by itself 3 or 4 or more times ? What if we divide 1 by i ? What if we divide 2 by 1+i ? Hey presto - you have invented complex numbers. Hamilton asked "what if we could divide one 3D vector by another ?" and invented quaternions. Cantor asked "what if there were a number larger than any finite number ?" and invented transfinite numbers. George Boole asked "what if 2=0 ?" and invented Boolean algebra.
- Physicists can play "what if ?" as well - "what if every fundamental particle were a one-dimensional vibratng loop in 11 dimensions" leads to string theory. The difference is that physicists are expected to provide experimental evidence that the results of their "what if" games bear some resemblence to the real world. Mathematicians have no such obligation - the results of their "what if" games only need to be self-consistent (in fact, even that is not essential; an inconsistent mathematical structure is possible, it is just not very interesting). Gandalf61 (talk) 10:59, 21 January 2010 (UTC)
- OK, OK, none of you applied scientists and fundamental scientists are better than each other. Comet Tuttle (talk) 18:19, 21 January 2010 (UTC)
- You might like the article on Illegal primes as well, number you should not use, don't even thnk about them! :) Dmcq (talk) 11:59, 21 January 2010 (UTC)
- People constantly invent new types of numbers by taking some subset of the primes and naming them after themselves, or giving them some cute name. I generally am for deleting the articles about them as nonnotable vanity articles, since they usually lack references other than the "inventor" posting about them in some recreational math blog. Edison (talk) 19:43, 21 January 2010 (UTC)
There are well defined numbers whose values are currently unknown, such as the 48th Mersenne prime. (Calling it that is imprecise as there may still be undiscovered Mersenne primes between the 39th and what we currently call the 47th. I suppose you could say that k such that M43,112,609 is the kth Mersenne prime is unknown, although we know that it must satisfy k >= 47.) Likewise, p, the smallest number of colors required to color any separation of a plane into contiguous regions such that no two adjacent regions have the same color, was not proven to be 4 until 1976; and n, the largest integer n such that an + bn = cn has a solution over the positive integers was not proven to be 2 until 1995. 124.157.247.221 (talk) 01:43, 22 January 2010 (UTC)
- Those are not "new" numbers, they would simply be discovered values for a specific case in number theory. You want a "new" number? How about the one George Carlin once reported on, called "bleen".[10] ←Baseball Bugs What's up, Doc? carrots→ 06:12, 22 January 2010 (UTC)
Could you harness the energy from an avalanche?
Hello,
Would it be possible to harness energy from the downward force of an avalanche? For example, would it be possible to install some sort of device (perhaps a turbine of some sort) at the bottom of steep mountains and generate energy from avalanches? Is there currently anything that works on these principles?
Thanks! I truly appreciate any help. --70.49.194.21 (talk) 05:40, 21 January 2010 (UTC)
- Anytime something is moving it is theoretically possible to harness that mechanical energy and turn it into electricity. However, it is rarely practical to do so. For your avalanche power generator, you would need (at least) 3 things-
- - some kind of ducted & self-cleaning turbine system
- - perfect geography
- - frequent heavy snowfall
- Even if you found some place on earth that was sufficiently steep & snowy enough, you'd still have to build a power plant that was both avalanche-proof AND could produce cheaper electricity than whatever else is being used in the area. All of this is a moot point, however, because avalanches are singular events and power consumption is constant. Generating many kilowatts of electricity for only several seconds at your multi-million dollar plant only 2 or 3 times a year isn't going to satisfy any customers! 218.25.32.210 (talk) 07:08, 21 January 2010 (UTC)
- Concur with 218. I Imagine that Mountainous areas also have areas of steady wind, or valleys that the wind tends to be funnelled thorough, so Wind Turbines, being an existing technnology may be a far better bet. --220.101.28.25 (talk) 07:55, 21 January 2010 (UTC)
- Is it possible? Well, yes - as a thought-experiment you could dig a deep hole at the bottom of the mountain - hang a large basket over the top of the hole with a rope - wrap the other end of the rope around the shaft of a generator and when the snow lands in the basket, it's pushed down into the hole - pulling on the rope and thereby spinning the generator. Or you could cover the mountainside in the path of the avalanche with piezoelectric panels and gather power as the snow lands on them. So yeah - it's certainly possible. But is it sensible? Useful? Practical? Hell no! The capital cost of what would realistically be a hideously expensive machine would be impossible to justify. Avalanches (by their very nature) are rare and hard to predict. Having a big chunk of machinery sitting there completely idle for years waiting on the offchance that an avalanche might hit it is just not economically reasonable. Also, avalanches happen in beauty spots - sticking a huge piece of industrial-scale machinery in the middle of that wouldn't be popular. So, no, this is really a non-starter.
- A better approach would be to wait until the snow melts and use the flow of water to turn a turbine...but we already do that - it's called hydroelectric power. SteveBaker (talk) 13:35, 21 January 2010 (UTC)
- Concur with 218. I Imagine that Mountainous areas also have areas of steady wind, or valleys that the wind tends to be funnelled thorough, so Wind Turbines, being an existing technnology may be a far better bet. --220.101.28.25 (talk) 07:55, 21 January 2010 (UTC)
- A minor nitpick, Steve. Avalanches don't have to be rare or particularly hard to predict. They just seem that way becuase a) we humans know where avalanches tend to occur, and don't build stuff there; and b) when we do build, work, or play in an avalanche area, we tend to employ engineering and maintenance strategies to avoid them doing damage. Avalanche-prone areas in developed countries regularly make use of tools ranging from mortars to helicopter-dropped explosives to trigger smaller avalanches in order to avoid building up large amounts of unstable snow. (Neat - we have an article on avalanche control.) I fully agree with the rest of your comment, though; converting the kinetic energy of a short-term, violent event into useful amounts of electricity is a non-starter. TenOfAllTrades(talk) 14:11, 21 January 2010 (UTC)
- Tides have tremendous force. I've often wondered if there were a way to get power from the ocean currents or from the wave action along shores. Some reversible turbine that generates power no matter if the wave is coming in or going out. But then it has to be fish proof too... I have no idea if anything like that is even worth a darn to consider. (how much power could we get from waves?) --Neptunerover (talk) 15:38, 21 January 2010 (UTC)
- Something like tidal power or wave power? DMacks (talk) 15:44, 21 January 2010 (UTC)
- Well slap me silly! Somebody's way ahead of me! Thanks for pointing that out. That's really cool. --Neptunerover (talk) 16:35, 21 January 2010 (UTC)
- It should be clarified that wave power has nothing at all to do with tides. It generates power from waves, which are produced by wind. --Tango (talk) 18:13, 21 January 2010 (UTC)
- Something like tidal power or wave power? DMacks (talk) 15:44, 21 January 2010 (UTC)
- Tides have tremendous force. I've often wondered if there were a way to get power from the ocean currents or from the wave action along shores. Some reversible turbine that generates power no matter if the wave is coming in or going out. But then it has to be fish proof too... I have no idea if anything like that is even worth a darn to consider. (how much power could we get from waves?) --Neptunerover (talk) 15:38, 21 January 2010 (UTC)
- It is of course possible but it is highly impractical. Flowing rivers are just so much more predictable. Plus, places where avalanches occur tend to be far away from cities that need power. Power disperses as it travels long distances through the wires. Vranak (talk) 17:24, 21 January 2010 (UTC)
- I've heard it is possible with alternating current to use transformers to step up the voltage to such that resistive losses are minimal when it is sent a long distance. But now that I think about it, direct current could also be transmitted at extremely high voltage for minimal resistive losses, now that electronic valves can be used to change it to and from AC. Edison (talk) 19:33, 21 January 2010 (UTC)
- No doubt, but what magnitude of avalanche would it take to reach 'extremely high voltage'? The type that would occur vary rarely, I think! Vranak (talk) 19:52, 21 January 2010 (UTC)
- Sounds like another question entirely? See alternating current, Transformer, Electric power transmission Electric power distribution, Electricity grid, Mains electricity. 2nd sentence, Yes 'extremely high voltage' conversion is possible, and is done for very long distance distribution, see Electric power transmission para. 4, SMPS (Switching Mode Power Supply), and High-voltage direct current (HVDC) --220.101.28.25 (talk) 21:14, 21 January 2010 (UTC)
- The question can be rephrased "What voltage causes this kind of avalanche?" Cuddlyable3 (talk) 16:27, 22 January 2010 (UTC)
- Southern California would presently be a good candidate for testing mudslide turbines. How much energy could be extracted from a 10 meter foot wall of mud, 100 m long, with a density of 1730 kg/m3 sliding down a 200 meter hillside at 10 meters/sec? Compare to snow with a density of 160 to 481 kg/m3 but probably sliding twice as fast. Unfortunately the turbine would have to be sturdily built at great expense and would operate very rarely. Plain old water at 1000 kg/m3 has the advantage of steadier supply at greater pressure from hydroelectric dams. Edison (talk) 16:50, 22 January 2010 (UTC)
Cryogenic eutectic exergy
Which eutectic system has the most efficient exergy at cyrogenic temperatures?
On a related note, is the amount of work required to cool and recoverable from cooling a eutectic system from 3 degrees to 2 degrees Kelvin the same or less than the amount of work required to cool and recoverable from cooling it from 2 degrees to 1 degree Kelvin? If less, how much less? 76.254.70.144 (talk) 06:30, 21 January 2010 (UTC)
- Use newtons law of cooling to determine the time taken : --123.237.193.11 (talk) 14:58, 21 January 2010 (UTC)
pressure gauges
on what basis the pressure gauges are decided to mount it on the line? —Preceding unsigned comment added by Ssanthamson (talk • contribs) 08:30, 21 January 2010 (UTC)
- What pressure do you mean: gas, oil, water, something else? Can you tell us more about thde line? Cuddlyable3 (talk) 10:06, 21 January 2010 (UTC)
- If de pressure can be dangrous den the pressure gauge is mounted on de line. —Preceding unsigned comment added by 79.76.218.43 (talk) 01:00, 22 January 2010 (UTC)
driving force for sigmatropic rearrangements
What exactly are the driving forces behind sigmatropic rearrangement reactions? Are they more affected by entropy considerations than enthalpy considerations, e.g. law of mass action plays a large role here? Can I just look at bond energies? John Riemann Soong (talk) 14:59, 21 January 2010 (UTC)
A Google search for "sigmatropic driving force" throws up a few links that suggest it's generally driven by the stability of the product. Hope this helps. Brammers (talk) 20:48, 21 January 2010 (UTC)
very small question
I found this information: "The human brain has been estimated to contain 50–100 billion neurons." I'm having difficulty finding out how many neurons there are in the entire human nervous system though. Does anyone know? --Neptunerover (talk) 15:26, 21 January 2010 (UTC)
- 50-100 billion is an enormous range. Most of the neurons in the body are in the brain (assuming that designation includes the brain stem). There are important and numerous neurons in the spinal cord, and important but less numerous neurons in the ganglions, and distributed as sensing organs in various tissues, however, they are unlikely significant in comparison to the aforementioned huge and widely distributed average. Half an order of magnitude allows for a lot of error. Tuckerekcut (talk) 16:21, 21 January 2010 (UTC)
- Neptunerover, Neuron quotes the same number. However, Brain says "The cerebral cortex of the human brain contains roughly 15– 33 billion neurons depending on gender and age", just to complicate the issue. Note "estimated" and "roughly".
- It certainly appears that for the brain and entire body:
- There is great variability and
- No one knows for certain. --220.101.28.25 (talk) 17:06, 21 January 2010 (UTC)
- The cerebral cortex is only part of the brain, so those articles are consistent. --Tango (talk) 18:15, 21 January 2010 (UTC)
- Neptunerover, Neuron quotes the same number. However, Brain says "The cerebral cortex of the human brain contains roughly 15– 33 billion neurons depending on gender and age", just to complicate the issue. Note "estimated" and "roughly".
- The indefiniteness comes from the fact that counting neurons is not easy. The numbers actually derive mainly from tiny granule cells of the cerebellum, which are estimated to number anywhere from 40 billion to 80 billion in humans. The next largest group of neurons is pyramidal cells of the cerebral cortex, which are most commonly estimated to number around 10 billion. All other groups of neurons are much smaller -- there is no other type that numbers even one billion. Thus the numbers basically derive from cerebellar granule cells plus cortical pyramidal cells, plus a couple billion more for everything else. Looie496 (talk) 19:02, 21 January 2010 (UTC)
In looking up nerve cell, it goes right back to neuron. So I guess every nerve is considered to be a neuron? They're all connected in the same system with the spinal cord and everything. Into the brain then, with each hemisphere connected to its own side? Counting all the nerve cells, could 1/2 trillion be a decent ballpark figure? Thanks --Neptunerover (talk) 19:44, 21 January 2010 (UTC)
- I will venture an inexpert opinion and say that if the human brain contains fifty to a hundred billion neurons, then the remaining somatic neurons will in no way shift that estimate significantly. For instance there likely won't be 20 billion neurons in the rest of your body put together, maybe 2 billion. But this is speculation. The brain is the seat of consciousness and the motor neurons won't likely add up to much. Correct me if I'm off the mark, anatomists! Vranak (talk) 19:49, 21 January 2010 (UTC)
- Yes, "nerve cell" redirects to neuron because it is the same thing, the two terms are synonyms. A nerve is not a group of cells, it is a bundle of axons that are generated by neurons. And yes, the total number of neurons outside the brain is a tiny fraction of 1% of the number inside the brain. Looie496 (talk) 19:55, 21 January 2010 (UTC)
- There is a wide variation in the volume and mass of brains in normal adult humans. An old paper in Nature (1897) reported that among 50 normal adult male Scotsmen (said to be "a highly civilised and admittedly intellectual people") the cranial capacity ranged from 1240 cc to 1770 cc. Among 23 Scotswomen, the cranial capacity ranged from 1100 cc to 1625 cc, the largest 48% greater than the smallest. Why assume that there is one "correct" number for the count of neurons, when the volume varies so widely? There were also those considered mentally defective who had cranial capacity half of the normal average. The brain size and likely complexity also increases dramatically from the infant to the adult. The brain size diminishes with extreme age and senile dementia. Edison (talk) 16:38, 22 January 2010 (UTC)
dianionic copper
I don't really get transition metal bonding. Firstly, why would copper be oxidised beyond +1 -- are the spin stabilisations simply not as strong? Also, wouldn't a -2 charge make copper start a new orbital? John Riemann Soong (talk) 15:38, 21 January 2010 (UTC)
Orgasm
Is it possible for a human to orgasm without manual stimulation of the genitals (penis / clitoris)? Are there drugs that induce orgasm? Can the mind be trained to orgasm on command?
- Yes. See the articles Nocturnal emission and Prostate massage. Some takers of Clomipramine, a drug marketed under the brand name Anafranil by Ciba Pharmaceuticals, have experienced yawn-induced orgasms as a side effect[11]. Cuddlyable3 (talk) 20:21, 21 January 2010 (UTC)
- Yes it is perfectly possible. If you were to break down all the orgasms ever experienced it would be but a minuscule portion, but it can and does happen. Vranak (talk) 20:22, 21 January 2010 (UTC)
- There are porn star women who claim to have trained themselves to orgasm on demand via concentration alone (i.e. with no manual stimulus or medication, etc.). I have no direct knowledge of whether those claims are verifiable or not. Dragons flight (talk) 21:17, 21 January 2010 (UTC)
- It is possible to train the mind. Wired magazine has an article about fMRI studies of the brain during orgasm. The scan has to be done while the subject is lying absolutely still inside a tube, which isn't really a good setting for people to have orgasms. To get the scans they used women who could trigger orgasms using their minds alone. Staecker (talk) 21:24, 21 January 2010 (UTC)
Unrelated banter and jokes
|
|---|
|
- Speaking from personal experience, the answer is yes, it's achievable, though definitely not easy. But I suppose with practice (like if I were a porn star^^) it could be done with less effort, less concentration, and in half the time! :-) Maedin\talk 07:46, 22 January 2010 (UTC)
- Personally, I think that manual stimulation does not mean what the OP (and the others here, apparently) thinks it means. Deor (talk) 12:23, 22 January 2010 (UTC)
- OK, define what you think it means. To me, it means direct contact with hands or some other object. ←Baseball Bugs What's up, Doc? carrots→ 15:31, 22 January 2010 (UTC)
- Personally, I think that manual stimulation does not mean what the OP (and the others here, apparently) thinks it means. Deor (talk) 12:23, 22 January 2010 (UTC)
- Bugs, I was trying to avoid making a snarky comment about popular conceptions of the sex lives of Wikipedians, but manual can mean only "with the hand(s)" in this context; and the original question, "Is it possible for a human to orgasm without manual stimulation of the genitals?" suggests a familiarity with one form of reaching orgasm to the exclusion of more, shall we say, interpersonal methods. Deor (talk) 17:11, 22 January 2010 (UTC)
- I wouldn't know anything about the private lives of wikipedians, other than my own. But I see "manual" as meaning any kind of direct genital stimulation, including vibrators, intercourse, or whatever. And some women apparently can, be it from diamonds or from being stimulated somewhere else on their ample endowments. ←Baseball Bugs What's up, Doc? carrots→ 02:37, 23 January 2010 (UTC)
- Bugs, I was trying to avoid making a snarky comment about popular conceptions of the sex lives of Wikipedians, but manual can mean only "with the hand(s)" in this context; and the original question, "Is it possible for a human to orgasm without manual stimulation of the genitals?" suggests a familiarity with one form of reaching orgasm to the exclusion of more, shall we say, interpersonal methods. Deor (talk) 17:11, 22 January 2010 (UTC)
- I would add that neither males or females need to use their hands to masturbate. To differing extents, fixed showerheads, fixed vibrators of various kinds, furniture which doesn't move easily and washing machines (both mentioned in masturbation for females although would also work for males to some extent) or just lying down on a soft surface (mentioned for males) could remove the need for hands. Of course manual stimulation primarily using the hands is the most common technique even more so for males and even with the others I mentioned the hands would usually be used and it would often be hard to avoid using them entirely particularly as they may get in the way (unless you're a double amputee) or you may inadvertedly use them for leverage, support etc. But I would say it's questionable if using hands to aide with these and other techniques (e.g. sex dolls) counts as manual stimulation if you don't touch an erogenous zone for stimulation, unless you also count sex as well which will often involve the use of hands by at least one partner. (And even with the aide of other things, most masturbation will I expect include some manual stimulation because there's little reason to avoid it; as I expect a fair amount of sex.) Nil Einne (talk) 02:26, 23 January 2010 (UTC)
- I wondered about this, the original post has two elements; one is asking after the lack of manual stimiulation, and the other is "drugs that induce" and "on command", both of which imply no voluntary stimulation, physical or otherwise. It seems unlikely to me that an orgasm can be experienced just like flipping a switch, but I wouldn't say it's impossible. I'd google it, but I don't think my employers would appreciate the content, :-) Maedin\talk 16:34, 22 January 2010 (UTC)
Dienogest
What kind of molecule is dienogest? What is its IUPAC name? What are its effects in human and mouse females?--Mparu (talk) 20:42, 21 January 2010 (UTC)
- Did you check out dienogest? Perhaps you can find your answers in that article and the sources from which it quotes. DRosenbach (Talk | Contribs) 20:52, 21 January 2010 (UTC)
- DRosenbach, you are right. I searched for dienogest using the italian version of Google, it usually displays the en.wiki article, but it did not this time. However I should have used directly the en.wiki`s search engine, sorry for this. The article shows the answers of several query asked above, thanks: I would be glad to have further hints about the side effects of dienogest in female metabolism, expecially in the field of neurology/psicology.--Mparu (talk) 09:20, 22 January 2010 (UTC)
Salt and water balance
The typical Western diet includes a lot of salt. If someone changed their diet to natural non-processed foods and thus consumed practically no salt for two or three days, would their body start urinating out excess water in an attempt to maintain the salinity of their bodies? 92.24.85.238 (talk) 21:15, 21 January 2010 (UTC)
- NOT an expert in this area, but excess added dietary salt generally causes water retention. It seems unlikely urination would increase in the circumstance suggested, nor for the reason suggested. See Salt#Health_effects and also Water retention (medicine). Fluid balance may also be of interest. --220.101.28.25 (talk) 21:51, 21 January 2010 (UTC)
- Yes. When people go on a diet, it is common to reduce salt intake (no french fries, salted nuts, saltines, potato chips, or other salty/fatty snacks). The reduction in salt intake commonly results in loss of "water weight" which gives an encouraging weight loss in the first few days. It is a reduction in retained water rather than a loss of fat deposits. It is commonly followed by a plateau, so the person decides the diet is not working and he/she goes back to overeating. If a healthy calorie restricted diet were continued, eventually the loss of body fat would show up as additional weight loss. This has always seemed like nature's way of getting us to abandon diets.
It is a pity the Unknown Person's links above are to pop books. 92.29.31.202 (talk) 21:05, 22 January 2010 (UTC)
- On the other hand, drinking too much water at once causes a depletion of blood salt content and creates water intoxication. ~AH1(TCU) 02:36, 23 January 2010 (UTC)
Pluto habitat for life in 7 billion year sun
Wow, this black blog page nearly consume 30 minutes when looking at it, but they show then way Titan and Europa might be in 5 billion years. Earler people have said at sun's expansion, Pluto can actually get nice and warm what makes people think this would happen?--209.129.85.4 (talk) 21:23, 21 January 2010 (UTC)
- Stellar evolution is a subject that is fairly well understood by modern science. Dauto (talk) 21:46, 21 January 2010 (UTC)
- I can't find anything relevant on that blog page (you've actually linked to a Google image search page, which doesn't help), but I can tell you this: In about 5 billion years the Sun will expand into a red giant. It will have much greater luminosity and the outer solar system will warm up. However, that will only last a few million years. After that, the sun will collapse into a white dwarf and everything will cool down again (I'm not sure what the luminosity of the white dwarf will be compared to the present sun, but it will certainly be dimmer than the red giant). During those few million years, parts of the outer solar system may well be a nice temperature for humans, but there is a problem - bodies like Titan are very small (Titan is a little under twice the mass of our moon). They can only hold on to the atmospheres that they have now because those atmospheres are very cold. If they warmed up, the atmospheres would quickly leak away. Living on Titan while the sun is a red giant would be, at best, like living on the Moon now. --Tango (talk) 23:17, 21 January 2010 (UTC)
It's worse than that. When the sun runs out of hydrogen fuel and starts burning helium before it does the red giant thing - it's going to emit a Helium flash during which time it could be thousands of times brighter than it is now - the solar wind will massively increase in strength - overwhelming our protective magnetosphere and bathing everything in hard radiation - and that will be fatal to life anywhere in the solar system. So even if things somehow got survivable for humans in these normally inhospitable places, we have no way to survive the transition. We need to be many lightyears away - settled on a planet in orbit around a much younger star long before that happens! SteveBaker (talk) 03:27, 22 January 2010 (UTC)
- Steve, your sequence is wrong. The helium flash is at the end of the red giant phase not the beginning. Dragons flight (talk) 04:27, 22 January 2010 (UTC)
- Hmmm - you're right. My bad. SteveBaker (talk) 18:42, 22 January 2010 (UTC)
- And the helium flash lasts a matter of seconds, so as long as you are on the night side of whatever body you are living on, you should be fine. The Earth will have long been abandoned as the Sun heats up over time and the seas boil in about 1 billion years so, so we'll most likely be living in airtight habits that don't really care when happens on the other side of the planet/moon. --Tango (talk) 04:47, 22 January 2010 (UTC)
- Well, "fine" so long as you don't need the atmosphere on the other side of the planet to still be there afterwards! SteveBaker (talk) 18:42, 22 January 2010 (UTC)
- Yes, I just said that... --Tango (talk) 20:14, 22 January 2010 (UTC)
- Well, "fine" so long as you don't need the atmosphere on the other side of the planet to still be there afterwards! SteveBaker (talk) 18:42, 22 January 2010 (UTC)
- Steve, your sequence is wrong. The helium flash is at the end of the red giant phase not the beginning. Dragons flight (talk) 04:27, 22 January 2010 (UTC)
- Wait, Tango, Isn't Pluto cover with ice (it is methane mainly) and it have gas inside it? But Pluto is smaller than our moon. This could happen. When a ice sublimes, it can create a body of greenhouse effect to build a atmosphere, same thing could hapoen to Europa (moon), depends on what gas the white ice is made of.. Sublimation (chemistry) is changing state from ice straight to gas. Tango and Steve seem to miss a point.--209.129.85.4 (talk) 20:43, 22 January 2010 (UTC)
- Yes, you probably would get outgassing as Pluto heated up, but you wouldn't end up with a decent atmosphere. You would have a few traces which would quickly dissipate. --Tango (talk) 23:55, 22 January 2010 (UTC)
Darwin
Wasn't Darwin racist? --70.129.184.174 (talk) 22:59, 21 January 2010 (UTC)
- From our article Charles Darwin: "Darwin did not share the racism common at that time: a point examined by the philosopher Antony Flew, who is at pains to distance Darwin's attitudes from those later attributed to him. Darwin was strongly against slavery, against "ranking the so-called races of man as distinct species", and against ill-treatment of native people." Comet Tuttle (talk) 23:04, 21 January 2010 (UTC)
- But the subtitle of his book refers to "the preservation of favored races", he referred to Africans and Australians as "savages", and evolution says that Africans have changed less than Caucasians and are thus more similar to monkeys (in fact, several illustrations about human evolution would depict Africans as the evolutionary link between ape and man). --70.129.184.174 (talk) 23:07, 21 January 2010 (UTC)
- I think the title means "race" in a more general way than just humans, as a subset of any species (the book isn't about humans, for the most part). "Savage" hasn't always had a pejorative meaning, it just means to opposite of "civilised". It is true that (sub-Saharan) Africans and Australians (before western colonisation, anyway) did not have civilisations. In modern usage, "savage" and "civilised" are used rather differently to the literal meanings used by Darwin. It is also true that Africans haven't changed as much as people that left Africa (change is hastened by new environments and small populations) so, I guess, they probably are more similar to other primates. The difference is tiny, though - they may be a few percent closer, at most. --Tango (talk) 23:28, 21 January 2010 (UTC)
- "It is true that (sub-Saharan) Africans and Australians (before western colonisation, anyway) did not have civilisations." No, its not true. See Great Zimbabwe for example. The original inhabitants of Australia also had a culture, just different from Westetrn culture. Your comment about Africans not changing as much as people who left Africa seems unlikely when you consider their whole genotype not just appearances. 92.29.31.202 (talk) 14:10, 22 January 2010 (UTC)
- Apparently I need to do more research into African civilisations. As for genetic change, I am confident about that - the genetic variation within people of African descent is significantly lower than between people from other continents. That suggests Africans have changed little since Homo sapiens started to leave Africa, while those that left have adapted to their environments. --Tango (talk) 15:11, 22 January 2010 (UTC)
- "It is true that (sub-Saharan) Africans and Australians (before western colonisation, anyway) did not have civilisations." No, its not true. See Great Zimbabwe for example. The original inhabitants of Australia also had a culture, just different from Westetrn culture. Your comment about Africans not changing as much as people who left Africa seems unlikely when you consider their whole genotype not just appearances. 92.29.31.202 (talk) 14:10, 22 January 2010 (UTC)
- Note that "savage" does not necessarily have a negative connotation. See noble savage. Also, go to http://embryology.med.unsw.edu.au/pdf/Origin_of_Species.pdf and search for the word "race". You'll see that Darwin always uses "race" to refer to types of animals. --Bowlhover (talk) 01:06, 22 January 2010 (UTC)
- Darwin didn't talk about human evolution at all in Origin of Species, but he did in his later books. "The favored races" has nothing to do with human races. --Mr.98 (talk) 14:46, 22 January 2010 (UTC)
- I think the title means "race" in a more general way than just humans, as a subset of any species (the book isn't about humans, for the most part). "Savage" hasn't always had a pejorative meaning, it just means to opposite of "civilised". It is true that (sub-Saharan) Africans and Australians (before western colonisation, anyway) did not have civilisations. In modern usage, "savage" and "civilised" are used rather differently to the literal meanings used by Darwin. It is also true that Africans haven't changed as much as people that left Africa (change is hastened by new environments and small populations) so, I guess, they probably are more similar to other primates. The difference is tiny, though - they may be a few percent closer, at most. --Tango (talk) 23:28, 21 January 2010 (UTC)
- But the subtitle of his book refers to "the preservation of favored races", he referred to Africans and Australians as "savages", and evolution says that Africans have changed less than Caucasians and are thus more similar to monkeys (in fact, several illustrations about human evolution would depict Africans as the evolutionary link between ape and man). --70.129.184.174 (talk) 23:07, 21 January 2010 (UTC)
- Just about anyone from the 19th century would be considered racist today. APL (talk) 23:39, 21 January 2010 (UTC)
- Just about any westerner, maybe. It's best not to generalise too far. There will be a few exceptions even then, though. However, attempts to classify and judge people from history by today's standards do usually end in disaster. For example, were Ancient Greeks engaging in pederasty homosexuals? --Tango (talk) 03:34, 22 January 2010 (UTC)
- By strict definition, yes. The question would be whether it was considered a bad thing, by the general public. ←Baseball Bugs What's up, Doc? carrots→ 04:08, 22 January 2010 (UTC)
- Usually, Bugs, the answer is given as "no", because what we mean by "homosexual"—e.g. someone who exclusively defines their sexuality as being attracted to someone of the same gender—is a concept that would have had no meaning to the Ancient Greeks. We define a lot of our own sexuality as an act of dividing people into neat categories ("straight, gay, bisexual"), whereas the Greeks simply did not self-define in that way, and did not consider pederasty to be something that influenced questions about sexual identity. (Somewhat similar to the idea amongst prisoners that homosexual behavior while confined to an all-male facility does not in fact make one a homosexual, but is just "how things are done" there.) To take categories of the present and brute-force them onto practices of the past does not enlighten, generally speaking. It's more than just a question of whether it was condoned or not, but whether these forms of categories make any sense. An analogy that plays up the point might be trying to take medieval medical concepts and forcing them on to modern medicine... you can do it, if you want, but it doesn't make any sense and won't get you any insight, because the systems do not match up in any way, even though they are each considered to be true and natural by those who practice them. --Mr.98 (talk) 14:22, 22 January 2010 (UTC)
- Ironically, terms like homo, hetero, and so on are Greek roots. They may not have described themselves that way, but if you went back through a time machine and defined those terms for them, they would have shrugged and said, "Yeh, I guess I am. So what?" ←Baseball Bugs What's up, Doc? carrots→ 15:28, 22 January 2010 (UTC)
- Well, maybe. I would suspect it just wouldn't be a category they'd find acceptable. Less "so what" and more, "Wait, you can't lump me in with people who abuse children—that's not what I do." Imagine some person from a terribly politically correct future came and declared that you were a mass-murderer because you ate meat, or a slave-owner because you had a dog. You'd not only say, "wait, no I'm not," you'd say, "these categories are not correct." --Mr.98 (talk) 01:24, 23 January 2010 (UTC)
- Ironically, terms like homo, hetero, and so on are Greek roots. They may not have described themselves that way, but if you went back through a time machine and defined those terms for them, they would have shrugged and said, "Yeh, I guess I am. So what?" ←Baseball Bugs What's up, Doc? carrots→ 15:28, 22 January 2010 (UTC)
- Usually, Bugs, the answer is given as "no", because what we mean by "homosexual"—e.g. someone who exclusively defines their sexuality as being attracted to someone of the same gender—is a concept that would have had no meaning to the Ancient Greeks. We define a lot of our own sexuality as an act of dividing people into neat categories ("straight, gay, bisexual"), whereas the Greeks simply did not self-define in that way, and did not consider pederasty to be something that influenced questions about sexual identity. (Somewhat similar to the idea amongst prisoners that homosexual behavior while confined to an all-male facility does not in fact make one a homosexual, but is just "how things are done" there.) To take categories of the present and brute-force them onto practices of the past does not enlighten, generally speaking. It's more than just a question of whether it was condoned or not, but whether these forms of categories make any sense. An analogy that plays up the point might be trying to take medieval medical concepts and forcing them on to modern medicine... you can do it, if you want, but it doesn't make any sense and won't get you any insight, because the systems do not match up in any way, even though they are each considered to be true and natural by those who practice them. --Mr.98 (talk) 14:22, 22 January 2010 (UTC)
- I intentionally didn't restrict my statement to westerners, but perhaps I'm just ignorant. I couldn't think of any society back then where most people wouldn't be considered racist by modern, western standards. APL (talk) 15:25, 22 January 2010 (UTC)
- By strict definition, yes. The question would be whether it was considered a bad thing, by the general public. ←Baseball Bugs What's up, Doc? carrots→ 04:08, 22 January 2010 (UTC)
- Just about any westerner, maybe. It's best not to generalise too far. There will be a few exceptions even then, though. However, attempts to classify and judge people from history by today's standards do usually end in disaster. For example, were Ancient Greeks engaging in pederasty homosexuals? --Tango (talk) 03:34, 22 January 2010 (UTC)
- The book to read to find out Darwin's racial views is not Origin of Species, but Descent of Man. Approximately half of the book considers the question of race directly. (The other half considers birds, mostly.) Darwin argues what was, for Victorian Britain, a pretty liberal argument: that all people are of the same descent (different races are not different species or even subspecies), that differences between physical appearances of races are largely due to sexual selection (that is, aesthetic preferences that had been bred over time) and not natural selection, and that for the most part the difference between civilization and savagery is a matter of culture. These were extremely contradictory views when compared against the mainstream Victorian physical anthropology, who would have been Darwin's natural scientific affinity (at the time Darwin wrote, the physical anthropologists were making extremely racist arguments, going so far as to argue that Blacks were a totally different species than Whites and perhaps not really human). Stephen J. Gould has pointed out that Darwin was about as racist as Abraham Lincoln—neither of them questioned for a minute that white people, on the average, were light-years beyond the darker races in terms of intelligence and ability, but the societies they lived in gave them no opportunity to see any other alternatives. Darwin spends some great effort in Descent of Man showing how even the most apparently erudite Europeans are only a few cultural jumps from the most destitute and hopeless savages—not exactly a claim for racial superiority.
- If someone was espousing Darwin's views from the 1960s onwards, they'd be considered racist. If they were espousing them before the 1960s in intellectual circles, they'd be considered fairly liberal-minded. Our definition of racist has radically changed in the last fifty years, and to call Darwin a racist is to completely miss that his arguments were, at the time, considered extremely anti-racist. Darwin was certainly not the most progressive thinker in all respects. But on the race question, for his time, he was pretty out there.
- His views, of course, can be mobilized to support a variety of different positions. There are plenty who used Darwinian-style arguments to justify racism and the idea of racial superiority. But Darwin did not personally share these notions for the most part. It is a token to the robustness of the theory that it provides ammunition to so many different causes, and I don't think we can lay all that at Darwin's lap. (And lest we forget, people have used the Bible to justify all kinds of racism as well—it doesn't mean that the Bible is itself racist.) --Mr.98 (talk) 14:15, 22 January 2010 (UTC)
- Conversely, it's equally invalid to argue "Darwin was racist , therefore
Goddiditthe theory of evolution is wrong". AndrewWTaylor (talk) 14:21, 22 January 2010 (UTC)- The argument, as I've seen it, is "Darwin was racist, Darwinism is racist, Darwinism is dangerous because it'll make your kids racist, don't teach Darwinism." Which is especially loopy given that modern teaching of evolution is usually about how it is extremely against any ideas of racial superiority. But it resonates well with a certain kind of voter, which makes it rather insidious. Unfortunately a complex explanation of the historical truth of it (as I've tried to sketch out above) is a little more complicated than such people are probably willing to listen to. --Mr.98 (talk) 14:31, 22 January 2010 (UTC)
- By the same argument, since Newton was an alchemist who did not believe in the Trinity, gravity must be an occult heresy. Gandalf61 (talk) 14:56, 22 January 2010 (UTC)
- Indeed, gravity is heresy, the truth is that the earth sucks. SteveBaker (talk) 18:02, 22 January 2010 (UTC)
- And they accuse me of dredging up old jokes. :)
- Indeed, gravity is heresy, the truth is that the earth sucks. SteveBaker (talk) 18:02, 22 January 2010 (UTC)
- By the same argument, since Newton was an alchemist who did not believe in the Trinity, gravity must be an occult heresy. Gandalf61 (talk) 14:56, 22 January 2010 (UTC)
- The argument, as I've seen it, is "Darwin was racist, Darwinism is racist, Darwinism is dangerous because it'll make your kids racist, don't teach Darwinism." Which is especially loopy given that modern teaching of evolution is usually about how it is extremely against any ideas of racial superiority. But it resonates well with a certain kind of voter, which makes it rather insidious. Unfortunately a complex explanation of the historical truth of it (as I've tried to sketch out above) is a little more complicated than such people are probably willing to listen to. --Mr.98 (talk) 14:31, 22 January 2010 (UTC)
- Conversely, it's equally invalid to argue "Darwin was racist , therefore
- Yes, we do! SteveBaker (talk) 18:28, 22 January 2010 (UTC)
- It's just good to see I'm not alone. :) ←Baseball Bugs What's up, Doc? carrots→ 02:29, 23 January 2010 (UTC)
- Yes, we do! SteveBaker (talk) 18:28, 22 January 2010 (UTC)
- I have a hard time believing that anyone with the radical, free-thinking ideas of Darwin would spend much time being vitriolic towards any particular race. Vranak (talk) 19:40, 22 January 2010 (UTC)
- Racism is not necessarily about vitriol. It can be mere indifference towards any race other than one's own; or a general sense that one's own is inherently superior, while all the others are inherently inferior, so inferior it's not worth even mentioning them by name. That sort of thing. Indifference is a far more toxic thing than active hatred, because indifference, not hatred, is the opposite of love. -- Jack of Oz ... speak! ... 01:59, 23 January 2010 (UTC)
January 22
Pelvic cavity capacity
Is there the same amount of space inside a mans pelvic cavity as in a womans? What is the capacity of spare space in liters (assuming empty bowel) —Preceding unsigned comment added by 79.76.218.43 (talk) 01:06, 22 January 2010 (UTC)
- The linked article might be a good starting point, although I didn't see where it answers your question directly. Based strictly on anecdotal observation, I would say it varies by individual. ←Baseball Bugs What's up, Doc? carrots→ 06:01, 22 January 2010 (UTC)
- See also Pelvis#Sexual_dimorphism, although again there's no direct answer. Neurotip (talk) 10:13, 22 January 2010 (UTC)
- It might be more straightforward to compare the openings rather than the volume, which would have to have the top and bottom specified, as well as the outer contours. Women need a large opening for childbirth, but a large volume is not so clearly needed for that purpose. In Iowa, "citizen soldiers" apparently need both a large pelvic volume and a large pelvic opening. The state seal specifies an image of a citizen soldier "with a plow in his rear." Edison (talk) 16:08, 22 January 2010 (UTC)
Why can't water freeze when salt is added?
Why can't water freeze when salt is added? —Preceding unsigned comment added by 71.194.113.40 (talk) 01:35, 22 January 2010 (UTC)
- It's not that it can't freeze, it's just the freezing takes place at a lower temperature than normal. This is referred to as Freezing-point depression. Truthforitsownsake (talk) 02:17, 22 January 2010 (UTC)
- Here's a non-wiki explanation of salt lowering the melting point.[12] And isn't the Arctic Ocean covered with frozen brine? ←Baseball Bugs What's up, Doc? carrots→ 02:22, 22 January 2010 (UTC)
- No, seawater at atmospheric pressure freezes by salt exclusion, so sea ice is mostly fresh water. Dragons flight (talk) 02:33, 22 January 2010 (UTC)
- I assume saltwater has to freeze at some point above absolute 0. Presumably it would be in the 10-to-20 degree area, the point where salting ice doesn't work? ←Baseball Bugs What's up, Doc? carrots→ 04:06, 22 January 2010 (UTC)
- If you try to freeze a deep body of salt water what you get is a layer of fresh water ice above a slightly salter body of water. That process of separating into fresh water ice and saltier brine is preferred until the brine gets substantially saltier than sea water. If you could isolate that brine, then you could eventually freeze it, but different ions freeze at different temperatures. It would take till around -35 C before the remnant solution of calcium and sulfate ions finally freezes and you are left with a true solid (though actually sulfate can push past -55 C if there aren't enough of the right kinds of cations present). Dragons flight (talk) 04:23, 22 January 2010 (UTC)
- So the specific answer to the OP's question is that it can freeze, at around -55 C at the lowest, which is about -67 F if I used the right formula. ←Baseball Bugs What's up, Doc? carrots→ 04:41, 22 January 2010 (UTC)
- No, -55 C is exactly -67 F. Cuddlyable3 (talk) 14:34, 22 January 2010 (UTC)
- See Freeze distillation: it's very hard to get ice with impurities in it. --Carnildo (talk) 00:45, 23 January 2010 (UTC)
Prilosec and Prevacid
Whats the simliarites and differences between two.
Basically, would like to know since my current prescription to generic prilosec is about to run out. At the same time I was told I had to see my doctor in order to get more of it. Which I totally not worth it in the long run since I have tried Prevacid in the past as well. The reason why I'm on Prilosec at the moment because insurance stopped covering Prevacid and had to switched to Prilosec at that time. Also, my parents are on Prilosec as well. One of them is in the same situation as me and has decided to take OTC Prilosec as the another one takes as well.
Believes that it for now.
Thank you, in advance. —Preceding unsigned comment added by Jessicaabruno (talk • contribs) 01:42, 22 January 2010 (UTC)
- Hi Jessica, we really can't answer this question because it almost certainly will constitute medical advice - even if those drugs may be over-the-counter medicine. You should definitely talk to your doctor - it is his or her job to explain these sorts of things to you. If you don't understand their explanation, keep asking them until you understand it. We can link you to the Prilosec and Prevacid articles, but you should not rely on Wikipedia for anything more than a very basic overview of these drugs - especially since we can't guarantee the accuracy of those articles. Nimur (talk) 02:25, 22 January 2010 (UTC)
Thank you, Nimur and didn't know that Wikipedia had a policy on this.--Jessica A Bruno (talk) 20:04, 22 January 2010 (UTC)
- In the U.S. a pharmacist will generally explain the ingredients in such medicines and their effects. Edison (talk) 15:59, 22 January 2010 (UTC)
- You might want to observe if you gulp some air while coughing, thus increasing the pressure. If so, you might want to tell your doctor just in case he forgets to ask about it. 95.115.144.18 (talk) 20:29, 22 January 2010 (UTC)
Genetic mutations, human electromagnetic fields and electronics
Some people say that electronic devices seem to malfunction more often or fail sooner when they're nearby. Is it theoretically possible that a person with the "right" genetic mutations could emit an electromagnetic field strong enough to damage devices or disrupt their functioning? NeonMerlin 02:46, 22 January 2010 (UTC)
- Yes, I expect so. Electric eels are capable of producing large electric fields, so it is certainly possible. It is ridiculously unlikely that that is the case, though. It would require a very large number of mutations, too many to occur in one person at any realistic probability, so it would take many generations to happen. Far more likely is that they are suffering from confirmation bias. --Tango (talk) 03:28, 22 January 2010 (UTC)
- No! Hell no! We'd be easily able to measure a field that strong - and we can't. Electric eels can do that - but they have these enormous, easily spotted organs in their bodies for doing that. They evolved those organs for a reason - why would humans evolve the ability to zap electronics? No - this is just another one of those ridiculous claims people make when they are sad little people with nothing remarkable to say about themselves who wish to feel important and special. If this were true, we'd know all about it by now. I'm sure the James Randi foundation would be happy to pay out a million dollars to anyone who could demonstrate this ability in a proper scientific test. Please tell this to people who claim to have this mysterious ability - and watch as their claims shrink to "well, sometimes it happens" - which is a clear case observer bias. SteveBaker (talk) 04:23, 22 January 2010 (UTC)
- There is a difference between a theoretical impossibility and something that is merely highly improbable. There is no law of physics that forbids humans that emit large EM fields, so the answer to the OP's question is "yes, it is theoretically possible". --Tango (talk) 04:54, 22 January 2010 (UTC)
- Well - theoretically in the sense that it's theoretically possible for someone to be born with blue and green striped skin and six heads. An electric eel has to use a dedicated organ that occupies 80% of it's body volume to produce the pulse it generates! Even that 500 volts at 1 amp wouldn't be anywhere close to enough to leap several feet through the air to zap a piece of electronics. Remember - electric eels live in salty, highly conductive water...but the trick that we're talking about here has to work through air which is a damned good insulator. You can put your phone in front of the 5,000 volts produced in a TV set and you're not taking ANY risk of damaging it! Even if some random human mutation were somehow to produce an organ able to do that - the odds of it happening are so spectacularly astronomically unlikely that we can be very confident that it won't happen over the life of the universe. Electric eels gained this ability through slow piece-by-piece evolution over millions of years - not in one might flook. We can also be sure that the person afflicted with this mutation wouldn't survive the self-induced shock after they first activated this organ. We can also be sure that this thing would occupy several cubic feet of body space - so it would be REALLY noticable. Someone with this ability would be a freak-show mutation - not just some ordinary looking person. Please - we get enough of this crap in the world - let's not extend any hope to the bozo's who claim this kind of crap. No, it can't happen - period. SteveBaker (talk) 05:32, 22 January 2010 (UTC)
- There is a difference between a theoretical impossibility and something that is merely highly improbable. There is no law of physics that forbids humans that emit large EM fields, so the answer to the OP's question is "yes, it is theoretically possible". --Tango (talk) 04:54, 22 January 2010 (UTC)
- Scientific method comprises formulation of hypotheses, such as the OP reported, followed by rational enquiry into what needs to be, and what can by experiment be, validated. Characterising an unproven hypothesis as crap claimed by bozo's who are "sad little people with nothing remarkable to say about themselves who wish to feel important and special" is not scientific. See the articles Theremin about an electronic device that can be affected by a person at a (short) distance and Radar about electronic devices that can be affected by secondary radiation from a person at a great distance. Light is electromagnetic radiation that when reflected from a person can in particular circumstances upset the function of a low-light camera. Cuddlyable3 (talk) 14:27, 22 January 2010 (UTC)
- So theramins and radar are driven by (to quote our OP) "a person with the "right" genetic mutations could emit an electromagnetic field"? No - theramins work by the change in capacitance of the air caused by the person and radar works by the person reflecting electromagnetic waves - not emitting them. Reflection of light is not emitting light - and you don't need genetic mutations to operate a theramin, or to be visible to radar. Nothing in your previous response has any bearing whatever on the OP's question. SteveBaker (talk) 17:58, 22 January 2010 (UTC)
- Scientific method comprises formulation of hypotheses, such as the OP reported, followed by rational enquiry into what needs to be, and what can by experiment be, validated. Characterising an unproven hypothesis as crap claimed by bozo's who are "sad little people with nothing remarkable to say about themselves who wish to feel important and special" is not scientific. See the articles Theremin about an electronic device that can be affected by a person at a (short) distance and Radar about electronic devices that can be affected by secondary radiation from a person at a great distance. Light is electromagnetic radiation that when reflected from a person can in particular circumstances upset the function of a low-light camera. Cuddlyable3 (talk) 14:27, 22 January 2010 (UTC)
- Another way to look at it is that the devices are nearby because they are used more frequently, and the greater usage might shorten their functional life. ←Baseball Bugs What's up, Doc? carrots→ 04:57, 22 January 2010 (UTC)
- One possible article is Electrokinesis (ability). But more likely would be that the person misused the item, gave the item an electrostatic discharge possibly by wearing charged nylon pants, or spilled a drink on it. Graeme Bartlett (talk) 08:25, 22 January 2010 (UTC)
- I read an account of a particular woman who could cause data processing equipment to malfunction. It was found to be due to electrostatic discharge due to the clothes/shoes she wore allowing a charge to build up. Plastic shoe soles would insulate more than leather soles. Walking across some carpets can build up several thousand volts of charge on a person, sufficient to make a large and painful spark when he touches something grounded. Such a discharge could damage many electronic devices. But a trash can dragged across the rug could also build up a static charge. Nothing special about it being a human. In an electronics lab I have visited, workers wear grounding electrodes to make sure their shoe soles do not insulate them from the floor, but which have some fuse capability so they would not carry a large current to electrocute the worker. There is nothing special about a human in relation to a Theremin. I expect a cabbage or a piece of aluminum foil or a bag of dirt moving near it could also affect the capacitance and cause an audible effect from a Theremin. My grandfather could never wear a wristwatch, because they stopped working, so he carried a pocket watch. I believe this was due to the amount or nature of his sweat rather than any electric effect. Edison (talk) 15:57, 22 January 2010 (UTC)
- Indeed, it's very possible to zap an electronic device with a static charge - but the OP says (a) "when they are nearby" - not necessarily touching or holding the device - which rules out anything but a really severe spark jumping an unlikely distance - and (b) that this is a "genetic mutation" that causes an electric field to be "emitted" - which doesn't cover merely accumulating static by wearing the wrong kinds of clothes and shoes because our genes don't control that. But even then, a piece of personal electronics like a cellphone or something isn't going to be zapped like that while it's in its case with all of the wires hooked up to the battery. Certainly humans can damage electronics - throwing them violently at the nearest brick wall will do that with a fair degree of reliability! That's not what we're being asked here. Can someone with a genetic mutation emit an electromagnetic field strong enough to destroy nearby electronics? To which the answer is a very definite "No!". Saying otherwise and throwing in random references to theramins (which are specifically DESIGNED to pick up the presence of a nearby human) does a disservice to our questioner by merely muddying the waters. SteveBaker (talk) 17:58, 22 January 2010 (UTC)
- The original poster was not specific about what "electronic devices" are malfunctioning, but another possibility is that the original complainant was talking about a radio or a baby monitor, and as you walk around the radio you certainly will block the signal in some places, possibly causing a lot of static, depending on the character and strength of the inbound signal. Alternatively, if the complainant is carrying a cellular phone, there's the "gallop" interference that is often heard on nearby speakers (including my computer speakers) every 5 or 10 minutes as the cell phone checks in with the local tower; maybe that's the "malfunctioning" that is being complained about. Not related to genetic mutation, except, I guess, for all the genetic mutations in the past that have led our bodies to be physically large enough to interfere with radio signals. Comet Tuttle (talk) 18:24, 22 January 2010 (UTC)
- Let me just point out that eels live in water, which is highly conductive. An electric eel out of the water is unlikely to have any noticeable effect on electronic equipment nearby. It would be like an AC outlet with nothing plugged into it. Looie496 (talk) 18:28, 22 January 2010 (UTC)
- And significantly, electric eels only live in salt water which is much more conductive than fresh water. The very few 'electric' fish that live in fresh water are only able to deliver relatively puny belts compared to electric eels. (See Electric skate, for example). SteveBaker (talk) 18:39, 22 January 2010 (UTC)
- The Electric eel lives in the Amazon and other rivers - fresh water. 92.29.31.202 (talk) 21:10, 22 January 2010 (UTC)
- And significantly, electric eels only live in salt water which is much more conductive than fresh water. The very few 'electric' fish that live in fresh water are only able to deliver relatively puny belts compared to electric eels. (See Electric skate, for example). SteveBaker (talk) 18:39, 22 January 2010 (UTC)
- Electronic devices can be quite sensitive, and humans can gather quite a lot of static electricity. When my mobile rings next to my computer mouse the later sends a couple of mouse-wheel-events. On times I don't need to actually touch the touch-pad of my notebook to move the mouse, it is sufficient to move my finger some distance above without contact. When we had to build circuits at university from TTL chips we always could test them previous to switching the real power on. I simply touched the plus and minus wires with the fingers of my left and right hand respectively (or the other way round, didn't make a difference). It was only a few mV but enough to reliably check the logic of the circuit. I don't know how it worked, but it worked reproducibly every time I tried. And no, I'm not joking. 95.115.144.18 (talk) 20:11, 22 January 2010 (UTC)
- Once, I took an alarm clock with an LCD display and barely any battery power left, and it appeared to become clearer when I placed it near me. I think I also posted this as a question on the RefDesk, and it's perhaps possible that the small variations in EMF fields had a small effect on the almost as small remaining battery energy. ~AH1(TCU) 02:21, 23 January 2010 (UTC)
immunity and cancer
cancer is caused by our own cells , so immunity of body does not effect it. is that so? or immunity of body effect the spreadin' of cancer. in todays date is there any cure of cancer except the lasers? are lasers efficient enough to kill cancer cells from root? I M NOT ASKING A MEDICAL ADVICE, its just zeal
- Have a good read of our lengthy cancer article. It's not a simple topic. Also there are many Management of cancer options available today which are discussed in that article, not sure i've ever heard of lasers being one of them. Vespine (talk) 04:04, 22 January 2010 (UTC)
- Immunity: no, that's not so. See Cancer immunology. Neurotip (talk) 10:08, 22 January 2010 (UTC)
- Have a good read of our lengthy cancer article. It's not a simple topic. Also there are many Management of cancer options available today which are discussed in that article, not sure i've ever heard of lasers being one of them. Vespine (talk) 04:04, 22 January 2010 (UTC)
Cancer arises from our own cells, not caused by our own cells. Immunity and other body responses is important in regulation of most types of cell growth and tumors including cancer suppression. There are many treatments that can cure cancer. Surgery can cure many cancers. Chemotherapy can cure some cancers. Radiation can cure some cancers. Most treatments of most serious diseases have some risk and some cost. The ratios of likelihood of cure to likelihood of harm vary enormously for different cancers, different people, different treatments. alteripse (talk) 23:00, 22 January 2010 (UTC)
- Research suggests that deoxycholic acid may have cancer-fighting capabilities. ~AH1(TCU) 02:16, 23 January 2010 (UTC)
Red dwarfs: proportion to all stars
According to the best current estimates (the current consensus), what percentage (range of percentage) of the stars in the universe are red dwarfs? —Preceding unsigned comment added by 63.17.40.163 (talk) 04:55, 22 January 2010 (UTC)
- Our article on red dwarf stars doesn't mention this, but there is a reference in Stellar classification#Class M. It gives a fraction of 76% (for main sequence stars in the solar neighbourhood). That tally only includes stars of absolute magnitude 16 or brighter; the proportion of Class M stars would rise further if dimmer stars were included. TenOfAllTrades(talk) 05:06, 22 January 2010 (UTC)
- Thank you -- I saw that figure, but thought perhaps the solar neighborhood (being not necessarily typical of all regions) might not be representative of the universe, which is the context of my question. Also, the article doesn't state what percentage of class M stars are red dwarfs, so there were two uncertainties. So: can anyone answer this regarding only red dwarfs, and in the context of the universe (instead of class M stars in the solar neighborhood)? —Preceding unsigned comment added by 63.17.40.163 (talk) 05:34, 22 January 2010 (UTC)
- Class M stars are red, and by numbers they would be almost all dwarfs, with just a few giants. If you count in the solar neighbourhood, you can make sure that you can see them. Are you interested in brown dwarfs? Graeme Bartlett (talk) 08:17, 22 January 2010 (UTC)
- So I guess the question is: Is the solar neighborhood representative of the universe in regard to the proportion of class M stars? If so, then approximately 75% ("almost all" of 76%) of all the stars in the universe are red dwarfs. If not, then what is the answer? —Preceding unsigned comment added by 63.17.45.118 (talk) 10:04, 22 January 2010 (UTC)
- Oh -- and the figure of 75% would have to be adjusted higher (a little? a lot?) because of this: "That tally only includes stars of absolute magnitude 16 or brighter; the proportion of Class M stars would rise further if dimmer stars were included." Incidentally, I'm asking this because the consensus is that red dwarfs would not have planets capable of nurturing intelligent life, so the fact that they are (apparently) at least 75% of all stars is relevant to the discussion of life in the universe. —Preceding unsigned comment added by 63.17.45.118 (talk) 10:17, 22 January 2010 (UTC)
- That used to be the consensus, but I've seen it challenged quite a lot in recent years. The habitable zone for a red dwarf is much closer in and much narrow, but it does exist. --Tango (talk) 12:31, 22 January 2010 (UTC)
- ...and of course that "habitable zone" is for "life as we know it" and not "life as we don't know it but could imagine" or "life as we don't know it and couldn't even imagine". Anywhere where there is a reasonable quantity of energy and matter, there could be some kind of life. SteveBaker (talk) 18:22, 22 January 2010 (UTC)
- It's relevant to the discussion of life, but proportion of habitable stars is only one parameter of the Drake equation. Given that there are at least four more parameters at which we can only guess the values (life, intelligent, signaling, duration), this one discussion shouldn't be enough to make or break any theories about extrasolar life. — Lomn 13:56, 22 January 2010 (UTC)
- Well, three of those four unknowns are not strictly relevent - the probability of there being extrasolar life is not the same thing as the probability of extrasolar intelligent life that is able and willing to talk to us right now! So if all you care about is whether there is extrasolar life at all then there are far fewer unknowns. We have a pretty solid idea of how many extrasolar planets there are. We'll soon be able to analyse the atmospheres of extrasolar planets and calculate what percentage are good for "life as we know it" and "life as we don't know it - but could imagine" - and at that point there is pretty much only one serious unknown - which is the probability of an abiogenesis event occurring (or the probability of a panspermia kind of event). Sadly, since we currently have no idea what our abiogenesis event was or whether panspermia is even a reasonable hypothesis, there is a big enough error bar on that number to make the result of the Drake equation come out to zero or infinity or anything between! Good news for science fiction authors - not so good news for the SETI folks! SteveBaker (talk) 18:22, 22 January 2010 (UTC)
- We have a pretty good idea about how many large planets there are. We're just getting started with Earth-sized ones. --Tango (talk) 00:01, 23 January 2010 (UTC)
- Well, three of those four unknowns are not strictly relevent - the probability of there being extrasolar life is not the same thing as the probability of extrasolar intelligent life that is able and willing to talk to us right now! So if all you care about is whether there is extrasolar life at all then there are far fewer unknowns. We have a pretty solid idea of how many extrasolar planets there are. We'll soon be able to analyse the atmospheres of extrasolar planets and calculate what percentage are good for "life as we know it" and "life as we don't know it - but could imagine" - and at that point there is pretty much only one serious unknown - which is the probability of an abiogenesis event occurring (or the probability of a panspermia kind of event). Sadly, since we currently have no idea what our abiogenesis event was or whether panspermia is even a reasonable hypothesis, there is a big enough error bar on that number to make the result of the Drake equation come out to zero or infinity or anything between! Good news for science fiction authors - not so good news for the SETI folks! SteveBaker (talk) 18:22, 22 January 2010 (UTC)
- That used to be the consensus, but I've seen it challenged quite a lot in recent years. The habitable zone for a red dwarf is much closer in and much narrow, but it does exist. --Tango (talk) 12:31, 22 January 2010 (UTC)
- Oh -- and the figure of 75% would have to be adjusted higher (a little? a lot?) because of this: "That tally only includes stars of absolute magnitude 16 or brighter; the proportion of Class M stars would rise further if dimmer stars were included." Incidentally, I'm asking this because the consensus is that red dwarfs would not have planets capable of nurturing intelligent life, so the fact that they are (apparently) at least 75% of all stars is relevant to the discussion of life in the universe. —Preceding unsigned comment added by 63.17.45.118 (talk) 10:17, 22 January 2010 (UTC)
coil spring life
when a coil spring is subjected to repeatative load, how long it will sustain for same load —Preceding unsigned comment added by 59.160.18.209 (talk) 09:04, 22 January 2010 (UTC)
- It's impossible to say without knowing the properties and dimensions of the material out of which the spring is made, not to mention the load itself.--Shantavira|feed me 12:09, 22 January 2010 (UTC)
- Springs made of Steel, Phosphor bronze or Beryllium copper exhibit linear-elastic behaviour i.e. they obey Hooke's law provided their material's yield strength is not exceeded. An example of a device which depends on steel's unchanging elasticity (quantified by Young's modulus) is a Tuning fork. The article Metal fatigue lists factors that could shorten coil spring life under repeated cycling. Two applications where this is critical are steel coil springs in automobile suspensions and valve gear.Cuddlyable3 (talk) 13:55, 22 January 2010 (UTC)
Calculating Pressure
I came across a sum in my textbook, which asks the reader to calculate the pressure at the bottom of a sealed cylindrical container fully filed with water which has been laid down on its side. The answer at the back is given as: 1/2ρgh+Ρ where P is air pressure at sea level. Can anyone explain why it's 1/2ρgh instead of simply ρgh? 117.194.224.21 (talk) 09:12, 22 January 2010 (UTC)
- Do you mean the average pressure on the circular end? Is the printed answer just an average? The pressure will be P at the top of the circular end, and P + ρgd at the bottom. Have I misunderstood the question? Dbfirs 09:20, 22 January 2010 (UTC)
- If I understand what you mean by "laid down on its side", then the depth of water is the diameter of the cylindrical. The given answer would then make sense only if the cylinder was twice as tall as it was wide (before being laid down), that is, h=4r. 124.157.247.221 (talk) 12:45, 22 January 2010 (UTC)
Golden syrup versus sugar
Which is the healthiest option as a sweetener in porridge: golden syrup, white sugar or brown sugar? Why?
Darkhorse06 (talk) 09:15, 22 January 2010 (UTC)
- It partly depends on what you regard as 'healthy'. Moderation in all things! It is unlikely to make any noticeable health difference unless you use these in large quantities. All these are rather high in calories, all are basically sugars of some type. Brown sugar is slightly less refined than white sugar.
See Also: sweetener, golden syrup, white sugar, brown sugar. You might also like to consider: Honey and Maple Syrup--220.101.28.25 (talk) 09:44, 22 January 2010 (UTC)- There's also (a recent invention), low GI sugar, at least where I live. [13]. However low in this case is 50, which isn't really that much less then the 65 for ordinary white sugar [14] and similar to what 220 said it's unlikely to really make that much of a difference. See also [15]. In particular, presuming you aren't using that much in you porridge it probably doesn't make that much an overall difference to the GI of the breakfast although at least you're consuming porridge rather then something like cornflakes or cocoa pops which likely have a high GI themselves. You may want to consider something like a banana or some other fruit instead of any normal sweetener Nil Einne (talk) 10:15, 22 January 2010 (UTC) That's Coco Pops (no 'a') on Wikipedia 'Cuz'. ;)) --220.101.28.25 (talk) 11:00, 22 January 2010 (UTC)
- I don't know enough about the subject, the benefits of honey may have been overstated in its article, as it is mostly simple sugar. 67.243.1.21 (talk) 15:45, 22 January 2010 (UTC)
- There's also (a recent invention), low GI sugar, at least where I live. [13]. However low in this case is 50, which isn't really that much less then the 65 for ordinary white sugar [14] and similar to what 220 said it's unlikely to really make that much of a difference. See also [15]. In particular, presuming you aren't using that much in you porridge it probably doesn't make that much an overall difference to the GI of the breakfast although at least you're consuming porridge rather then something like cornflakes or cocoa pops which likely have a high GI themselves. You may want to consider something like a banana or some other fruit instead of any normal sweetener Nil Einne (talk) 10:15, 22 January 2010 (UTC) That's Coco Pops (no 'a') on Wikipedia 'Cuz'. ;)) --220.101.28.25 (talk) 11:00, 22 January 2010 (UTC)
- It's mostly sugar - and pound for pound it must have almost identical calories to sugar - but what makes it seem worse is that a level teaspoonful of sugar has an awful lot of air in it because of the large crystals - but a level teaspoonful of honey is all honey. SteveBaker (talk) 17:40, 22 January 2010 (UTC)
Heroin addiction
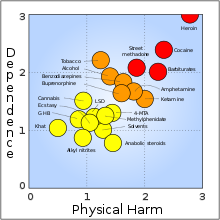
How many injections of heroin would it take for a new user to become addicted?
- In some cases, one. -- Aeluwas (talk) 09:50, 22 January 2010 (UTC)
- Yes, it would likely vary betweeen people. See Heroin, addiction and drug addiction, especially the Addictive potency section for more data. --220.101.28.25 (talk) 09:58, 22 January 2010 (UTC)
- Definitely, as little as one is enough in many cases. I know a guy who was intelligent, successful, etc who did the "I'll just try it once to see what it's like" thing - ten years later, he's still a total mess. That stuff is evil. SteveBaker (talk) 17:37, 22 January 2010 (UTC)
- Isn't it even possibly deadly on the first injection, for example if one has a low tolerance for drugs in general? The sad case of your friend sounds like something Garrison Keillor said once, about people "who make a bad decision and then stick with it." ←Baseball Bugs What's up, Doc? carrots→ 18:06, 22 January 2010 (UTC)
- It isn't possible to become physiologically addicted to heroin after a single exposure, it takes dozens at least. Psychological addiction is a different matter, but that's very difficult to quantify. The data show that physiological addiction is quite a bit more rapid for fast-acting psychostimulants such as crack cocaine than for heroin -- the flip side is that the withdrawal symptoms produced by heroin addiction are probably the most unpleasant of any type of drug. Looie496 (talk) 18:19, 22 January 2010 (UTC)
- Our Heroin article only alludes to answers of this simple question; could a knowledgeable editor add the answer to that article? Comet Tuttle (talk) 18:31, 22 January 2010 (UTC)
- (ec) Interesting...but of course what starts out as 'merely' a psychological addiction will pretty soon become a physiological one if not handled immediately. Having seen it happen, it's hard to believe that what made him go back for another fix was purely psychological...but if that's what it takes then I've gotta say that even intelligent, well-grounded, happy people can get psychologically addicted after the first shot. What the underlying cause of taking the second shot is - I have no clue. SteveBaker (talk) 18:34, 22 January 2010 (UTC)
- The trouble with psychoactive compounds is that the distinction between physical dependence and psychological dependence is
mootblurry - physiological effects manifest as perturbations of neurochemistry (among other physical dependence effects). The extent to which neurochemical dependence is separate from psychological dependence is hard to clearly define. There's no shortage of research on this, and it isn't clear to me that a scientific consensus exists. Nimur (talk) 18:44, 22 January 2010 (UTC)
- The trouble with psychoactive compounds is that the distinction between physical dependence and psychological dependence is
- (ec) Interesting...but of course what starts out as 'merely' a psychological addiction will pretty soon become a physiological one if not handled immediately. Having seen it happen, it's hard to believe that what made him go back for another fix was purely psychological...but if that's what it takes then I've gotta say that even intelligent, well-grounded, happy people can get psychologically addicted after the first shot. What the underlying cause of taking the second shot is - I have no clue. SteveBaker (talk) 18:34, 22 January 2010 (UTC)
- I don't know who posted that image from the Nutt et al paper, but it should be noted that the diagram is slightly flawed since the addictivity of cocaine doesn't take crack cocaine into consideration, which is possibly more psychologically addictive than heroin. Also, the methodology of that study isn't as scientific as one would assume for a Lancet paper. --Mark PEA (talk) 20:16, 22 January 2010 (UTC)
- I have seen a bbc documentary created from the results of that study, and they claimed a difference in effect between crack and powder cocaine could not be established.
- I grabbed it from our Heroin article and pasted it there — insert obligatory hectoring to be bold and go fix it or challenge its inclusion in that article, etc. Comet Tuttle (talk) 21:20, 22 January 2010 (UTC)
Speed
What is the maximum attainable speed, Please? —Preceding unsigned comment added by 123.237.193.11 (talk) 19:19, 22 January 2010 (UTC)
- The maximum for what? Cycling records, Water speed record, Land speed record, Motorcycle land speed record? Assuming you mean the fastest possible speed for anything, then why not read Speed, in which you will find the answer. Fences&Windows 19:36, 22 January 2010 (UTC)
- It's just below the speed of light ("c"). As mentioned in the Upper Limit on Speeds section, it would take an infinite amount of energy to accelerate an object with mass to the speed of light. Since you don't have an infinite amount of energy available, you can get closer and closer by continuing to accelerate, but you'll always be just a little slower than c. Comet Tuttle (talk) 19:33, 22 January 2010 (UTC)
- It's not only below the speed of light. You can actually reach the speed of light. You only need to find an adequate amount of anti matter and transform yourself to light.95.115.144.18 (talk) 19:47, 22 January 2010 (UTC)
- I thought we didn't do people's homework for them. Fences&Windows 19:38, 22 January 2010 (UTC)
- This doesn't sound at all like a homework question to me. Thanks for the mention of the Speed article; I just corrected the intro where it had been claiming that matter can reach c. Comet Tuttle (talk) 19:43, 22 January 2010 (UTC)
- This all sounds kind of familiar, but I'll ask anyway: photons travel at the speed of light, in part because they are "nearly" 0 mass. I might argue that they are not exactly 0 mass, just very small. So, is the speed of light really the upper limit for speed, or is it some other number that's just slightly more than the speed of light? Or is it more like limit formulas, such as 1/n, whose limit is 0 as n approaches infinity, but never actually reaches 0? Ya follow? ←Baseball Bugs What's up, Doc? carrots→ 20:06, 22 January 2010 (UTC)
- This doesn't sound at all like a homework question to me. Thanks for the mention of the Speed article; I just corrected the intro where it had been claiming that matter can reach c. Comet Tuttle (talk) 19:43, 22 January 2010 (UTC)
- Bugs, photons are presently understood to have exactly zero rest mass (that is, what we conventionally consider "mass"). There's no "nearly" about it (in absence of some verifiable and reproducible experimental result that suddenly upsets a lot of physics). c is the upper bound, which can be reached in the idealized "light in a vacuum" state, though this is never fully found in nature. Note, though, that "speed of light" becomes confusing terminology here -- if photons were found to have mass, and thus traveled at less than c, the photon's speed would be the "speed of light". In particular, the upper bound of c is borne out in quantum entanglement, which though resulting in changes that appear to propagate at speeds greater than c (perhaps immediately), does not transmit information. For transfer of information to work with quantum entanglement (quantum teleportation is one such application), a classical (i.e. c-limited) communication is also required. On the other hand, the notion of the tachyon as an FTL particle is still floated about, though it's presently considered highly unlikely to exist in a causality-breaking form. — Lomn 20:31, 22 January 2010 (UTC)
- I'm not so sure that they literally have 0 mass, but it doesn't really matter, because as far as we know, the speed photons travel (in reference to whatever medium they are in) is the fastest known velocity of any object within that medium (be it water, vacuum, or whatever). So if the upper bound is slightly higher than c, it doesn't really matter as it's unattainable. ←Baseball Bugs What's up, Doc? carrots→
- Bugs: PLEASE stop guessing - we all make mistakes - but we really do work hard not to guess. Before you answer, either be very sure you know the answer - or look it up. Look up the Lorentz transform in this handy dandy encyclopedia we have on the interweb and you'll see: . That nice Mr Lorentz said that at some speed 'v', the mass of the object is it's rest mass multiplied by gamma...things get heavier as they are accellerated towards the speed of light. So if you know the rest mass and the speed - you can figure out the relativistic mass. Now, if v is exactly equal to c (ie, if the object is moving at exactly the speed of light) then v2/c2 is exactly 1.0 - so gamma is one divided by the square root of zero...one over zero is either undefined (if you're a mathematician) or infinity (if you are a physicist) - so at exactly the speed of light, an object would become literally infinitely heavier than it was at rest. That means that it's mass would be literally bigger than the mass of the entire universe. That's not going to happen. However, we know that the photon does move at exactly the speed of light - and it doesn't have an infinite mass - it has a small but perfectly measurable relativistic mass while it's moving at the speed of light. So we can calculate the rest mass of the photon by dividing its relativistic mass by Lorentz's gamma factor. But if it's moving at the speed of light - then gamma is infinity and its rest mass is it's fairly small relativistic mass divided by infinity. Any number divided by infinity is...zero. So the rest mass of the photon MUST be precisely zero...not a teeny tiny bit above zero...it has to be EXACTLY zero. Now, when you say "it doesn't matter if it's a tiny bit above c" - you have to consider what you just said. If v is even the tiniest bit bigger than c then v2/c2 is just a hair bigger than 1.0 - but the number inside the square root of the lorentz transform is 1-v2/c2 - so that would be a negative number. The gamma factor would be one divided by the square root of a negative number...which is a complex number - and complex number never, EVER show up as real world quantities. Hence, speeds of even the most microscopic amount above c are as clearly impossible as basic arithmetic. It's not even something to discuss. SteveBaker (talk) 03:13, 23 January 2010 (UTC)
- As Lomn correctly explained above, the zero mass of photons is not a speculation, but an experimentally verified result. If there were a non-zero mass, even a very tiny one, it would have implications that contradict observation. They are massless particles and this theoretical principle is part of the Standard Model. Nimur (talk) 21:19, 22 January 2010 (UTC)
- It is not the case that, in every medium, light travels faster than anything else. See Cherenkov radiation for what can happen when things go faster than the local speed of light. Algebraist 21:02, 22 January 2010 (UTC)
- According to that article, there is a type of electromagnetic radiation that travels faster than "light" in that particular medium. However, "light" is a form of electromagnetic radiation. Unless they're restricting "light" to mean visible light, which is merely a subset of all the possibilities for electromagnetic radiation. Right? ←Baseball Bugs What's up, Doc? carrots→ 02:52, 23 January 2010 (UTC)
- I'm not so sure that they literally have 0 mass, but it doesn't really matter, because as far as we know, the speed photons travel (in reference to whatever medium they are in) is the fastest known velocity of any object within that medium (be it water, vacuum, or whatever). So if the upper bound is slightly higher than c, it doesn't really matter as it's unattainable. ←Baseball Bugs What's up, Doc? carrots→
- Bugs, photons are presently understood to have exactly zero rest mass (that is, what we conventionally consider "mass"). There's no "nearly" about it (in absence of some verifiable and reproducible experimental result that suddenly upsets a lot of physics). c is the upper bound, which can be reached in the idealized "light in a vacuum" state, though this is never fully found in nature. Note, though, that "speed of light" becomes confusing terminology here -- if photons were found to have mass, and thus traveled at less than c, the photon's speed would be the "speed of light". In particular, the upper bound of c is borne out in quantum entanglement, which though resulting in changes that appear to propagate at speeds greater than c (perhaps immediately), does not transmit information. For transfer of information to work with quantum entanglement (quantum teleportation is one such application), a classical (i.e. c-limited) communication is also required. On the other hand, the notion of the tachyon as an FTL particle is still floated about, though it's presently considered highly unlikely to exist in a causality-breaking form. — Lomn 20:31, 22 January 2010 (UTC)
When a body moves at a speed say .8c and another body at .5c (In opposite directions) Then the relative speed between them exceeds C while the maximum speed attainable is C .Doesn't this contradict the above statement PS this Isn't a homework 123.237.193.11
- Don't forget time dilation! Once who was traveling around at a close enough speed of light can have time run 1,000x slower for him, so that he perceives himself traveling at 1000c (even though only going at 0.99999c) Googlemeister (talk) 20:19, 22 January 2010 (UTC)
- Yes, that was a fundamental to one of Einstein's theories. Although in an absolute sense those two objects are speeding away from each other (or let's say from their common point of origin) at a net of 1.3c, from their own viewpoint they are not. Basically, nature corrects for this apparent paradox. ←Baseball Bugs What's up, Doc? carrots→ 20:31, 22 January 2010 (UTC)
- Yes on time dilation, no on perceiving 1000c (or anything greater than c). Every frame of reference is consistent with c being the maximum speed. Various tricks might be shown as to how a traveler might conclude that he's gone 1000c but they break down when shifts in frames of reference are taken into account. For example, consider the spaceship that travels from Earth to a star 1000 light years away, experiencing 1 year on its local clock. This is the sort of thing you might say equates to "1000c", right? But consider: the radio pulse broadcast from Earth simultaneous to the departure of the ship won't arrive 999 years later; it's already come and gone by the time the ship gets there. — Lomn 20:44, 22 January 2010 (UTC)
- (after edit conflict) @Googlemeister: Time is perceived to run at "normal rate" whatever the speed of travel, so no traveller can perceive himself to be travelling at 1000c. What he perceives is length contraction of objects not travelling at the same speed. (I suppose in some circumstances, that might amount to the same thing.)
- @BaseballBugs: A wrong calculation by a "stationary" observer would add 0.8c to 0.5c and wrongly conclude that the relative velocity was 1.3c. This faulty calculation would not be supported by the observations of either of the travellers. Dbfirs 20:48, 22 January 2010 (UTC)
- Precisely. That observer might be standing on the point of origin. He would see them pulling away at their respective speeds and conclude they were receding from each other at faster than light speed. But the ones receding wouldn't see it that way. ←Baseball Bugs What's up, Doc? carrots→ 20:54, 22 January 2010 (UTC)
- Yes, perhaps I misunderstood what you intended by "absolute sense". It is the faulty assumption about how to add speeds that creates the paradox. I suppose it is true to say that, in the frame of reference of the stationary observer, the rate of increase of separation of the travellers is 1.3c, but this cannot be interpreted as a relative speed observable by either of the travellers. Dbfirs 21:23, 22 January 2010 (UTC)
- Precisely. That observer might be standing on the point of origin. He would see them pulling away at their respective speeds and conclude they were receding from each other at faster than light speed. But the ones receding wouldn't see it that way. ←Baseball Bugs What's up, Doc? carrots→ 20:54, 22 January 2010 (UTC)
Quantum mechanics and probability
Hello again, and thank you in advance. As I always say when I ask these stupid questions, I am here as an amateur philosopher and only have a relatively superficial understanding of physics, and a slightly better but still probably inadequate understanding of maths, so you'll probably have to talk down to me a fair bit lol. But I'm curious anyway, and would appreciate it very much if you could aid my understanding.
My question is about the nature of probabilities in quantum mechanics. When there is something uncertain (like beta decay or wave function collapse or some other thing I kind of know about but don't really understand), is the probability of this uncertain thing occurring something that is known for sure? Like we might not know when a nucleus will emit an electron, but do we know at all times the probability that it will emit an electron?
That's a specific example but I'm asking the question generally. In quantum mechanics, are probabilities always certain quantities?
Thanks much! Dan Hartas (talk) 19:20, 22 January 2010 (UTC)
- No the probabilities may or may not be correct they are not always certain quantities . see Interpretation of quantum mechanics —Preceding unsigned comment added by 123.237.193.11 (talk) 20:19, 22 January 2010 (UTC)
I'm not sure I understand, I think maybe I didn't ask the question right. I wasn't asking about whether there was disagreement on the actual values or mechanisms of these probabilities, I was wondering whether science sees them as even having actual values. I'm talking specifically in terms of the Copenhagen interpretation I think. Dan Hartas (talk) 20:40, 22 January 2010 (UTC)
- If I understand the question correctly... (and assuming I understand this aspect of QM correctly)... let's take an example of something that is considered fundamentally probabilistic according to QM, like the decay of a nucleus of something radioactive. For any individual atom, when it will decay is fundamentally unknowable. I am not even sure if it can be expressed in a sense of "in every second, there is a 1/million chance that this could decay," but maybe someone can clarify that. However, taken as a group, the probabilities average in directly predictable ways according to the stability of the substance—thus we have half-lives, which are pretty iron-clad expressions of the rate of decay over a relatively large number of atoms. The half-life is not an expression of the likelihood of any given sample to decay in that period, but an observed expression of what that rate of decay will be, if that distinction makes sense. (The half-life article discusses this better terms than I am, I recognize.) --Mr.98 (talk) 00:39, 23 January 2010 (UTC)
- And as something else possibly relevant... if you are interested in how weird probabilities can get in QM, I find Bell's theorem and its experiments to be pretty interesting food for thought. It basically rules out the possibility of hidden variables—e.g., the idea that the uncertain aspects of QM are not being secretly carried around by the particles, invisible to the observer but "known" to the particles themselves (or known in a "God's eye view"), but are, in fact, ontologically uncertain. (There are other interpretations of Bell's theorem, to be sure, but that it rules out local hidden variables seems to be the consensus, as I understand it. I am not a physicist, however!) --Mr.98 (talk) 00:43, 23 January 2010 (UTC)
- A quantum mechanical system is always in a definite state, described by a state vector. When time passes that state evolves (in the original nonrelativistic quantum mechanics) according to the hamiltonian of that system as described by the Schrödinger equation. The hamiltonian can be deduced from the laws of physics. The initial state must be measured or set up; it is usually impossible to know entirely. If however both are known, the future state vectors of the system are entirely determined by this.
- The Copenhagen interpretation now says, that whenever a property of the system is measured, the state vector of the system is projected (collapsed) onto one of the base states corresponding to the possible measurement results. The square of the length of the projection is proportional to the probability for that measurement result. So if the initial state, the hamiltonian, the amount of time passed and the base vectors of the measurement are known, the probabilities for each measurement can be deduced from this.
- Other interpretations give different answers. Hidden variables theories will claim that the probability for one measurement result is one and zero for all others and the full initial state vector cannot be known. Everett claims that the probabilities are all one; the state vector never collapses, it only couples with the measurement device to form a single quantum mechanical state which contains amplitudes for all measurement results. Mr. Coleman explains this way better than I ever could: http://media.physics.harvard.edu/video/index.php?id=SidneyColeman_QMIYF.flv —Preceding unsigned comment added by 80.226.1.7 (talk) 03:05, 23 January 2010 (UTC)
About human evolution
Starting from the article History of the Americas and found somewhere the theory that humans could have migrated to the Americas 40k years earlier than they did because siberia was infested by spotted hyena. This set my mind wondering and left me with two questions:
How long would human populations need to evolve without contact with each other to 1) not being able to genetically intermix any more, and, 2) not being susceptible to the other ones endemic diseases? Well, I know, it depends, but are there any theories or estimates out there? 95.115.144.18 (talk) 19:39, 22 January 2010 (UTC)
- I saw a TV show recently that said that homo sapiens and the neanderthals probably came from common ancestors in Africa. It's possible they could still have interbred. But if you can find an estimate on how long ago those branches split, that will give you a rough minimum figure - which I would guess is at least in the hundreds-of-thousands of years. Evolution among larger creatures is slow. But nature has had all kinds of time. ←Baseball Bugs What's up, Doc? carrots→ 20:02, 22 January 2010 (UTC)
- Why does the size of the creatures matter? Surely it is the length of a generation (that is, the average age of reproduction) that matters. There does seem to be a correlation between size and lifespan, but not a particularly strong one. --Tango (talk) 20:12, 22 January 2010 (UTC)
- Yes, rate of reproduction. Bacteria and viruses, and also insects, reproduce quickly, so mutation and evolution is way much faster in those critters. And they happen to be very small. ←Baseball Bugs What's up, Doc? carrots→ 20:25, 22 January 2010 (UTC)
- Why does the size of the creatures matter? Surely it is the length of a generation (that is, the average age of reproduction) that matters. There does seem to be a correlation between size and lifespan, but not a particularly strong one. --Tango (talk) 20:12, 22 January 2010 (UTC)
- Not being at all susceptible to the other's diseases would take a very long time - we can catch diseases from birds (see Bird flu) and we separated from them hundreds of millions of years ago. --Tango (talk) 20:12, 22 January 2010 (UTC)
- Doesn't the virus responsible for spreading bird flu need to be a mutated version in order to infect humans though? Otherwise there would have been a much much higher number of people who catch bird flu. Googlemeister (talk) 20:17, 22 January 2010 (UTC)
- The rate of mutation would be much faster in bugs that reproduce frequently, as suggested by Tango in his adjustment of my earlier comment. ←Baseball Bugs What's up, Doc? carrots→ 20:27, 22 January 2010 (UTC)
- Chimps are a somewhat better analog, because there are some diseases that we still have the same receptors for, despite 6 million years of evolutionary distance. We are too distant to reproduce with them (probably), but not too distant to share some of the same diseases. --Mr.98 (talk) 23:33, 22 January 2010 (UTC)
- Back in the 80s, it was theorized that AIDS originated among the apes. The ever-outspoken Frank Zappa said, "What I'd like to know is, who's fluking those monkeys???" ←Baseball Bugs What's up, Doc? carrots→ 02:23, 23 January 2010 (UTC)
- Doesn't the virus responsible for spreading bird flu need to be a mutated version in order to infect humans though? Otherwise there would have been a much much higher number of people who catch bird flu. Googlemeister (talk) 20:17, 22 January 2010 (UTC)
longjack
is longjack and Tongkat Ali the same thing? and does longjack contain all this: Eurycoma Longifolia Jack / Tongkat Ali / Malaysian Ginseng / Pasak Bumi? —Preceding unsigned comment added by 67.246.254.35 (talk) 19:59, 22 January 2010 (UTC)
- Yes they are but Muhammed Ali is different Tongkat Ali= Eurycoma longifolia 123.237.193.11 —Preceding unsigned comment added by 123.237.193.11 (talk) 20:13, 22 January 2010 (UTC)
Consumer Medication Guides
What should I look for?
Thinking about getting updated one because the current one that I have is Worst Pills, Best Pills. It is good, but it doesn't have everything that I'm looking for. At the same time it last update was 5 years ago and that's the one that I have. —Preceding unsigned comment added by Jessicaabruno (talk • contribs) 20:08, 22 January 2010 (UTC)
life on other planets
I was reading a question that involved red dwarf stars and the drake equation and a thought occurred to me. Given that all life as we know it is carbon based, and seemingly dependent upon either O2 or CO2 for life, is it possible to determine what the odds are that life in other forms exists within our solar system, say floating around 100 miles into Jupiter, or bacteria sized organisms oozing around on Venus, considering that we have not explored these areas which would have had a completely different evolutionary path? It does not seem like we have a feasible way to determine that life does not exist in those locations considering that any life we can experiment on would be adapted to live on our planet, not in those other areas. Googlemeister (talk) 20:15, 22 January 2010 (UTC)
- I have a hunch there's an article that would explore that topic. You could start with Life. Also, for that matter, it's possible we ourselves have introduced our own microbes to those areas where we have sent our various satellites and rovers - which might be able to adapt - and might even consume existing life forms there - or merge with them. ←Baseball Bugs What's up, Doc? carrots→ 20:20, 22 January 2010 (UTC)
- We have an article on SETI. Scientists do not rule out the possibilities of life - even life which is biochemically similar to earth life - within our own solar system. The probability of it existing is low, and the probability we might find it, even it if it does exist, is even lower - but it is part of the motivation for the study of planets and moons within our own solar system. One class of targets in recent years have been the moons of Saturn and Jupiter. You might like to read Extraterrestrial life#Direct search, Extraterrestrial life#Extraterrestrial life in the Solar_System, and the proposed Terrestrial Planet Finder for extrasolar planets. Nimur (talk) 21:24, 22 January 2010 (UTC)
- Carbon is uniquely capable of producing a wide variety of complicated compounds with properties that are really conducive to constructing living things - there are good chemical reasons for supposing that life elsewhere would have to be carbon-based. That in turn puts limits on the temperature range over which life is reasonable - and it's a small step from that to requiring there to be liquid water. But I have to STRONGLY disagree with Baseball Bugs that our microbes "might even consume existing life forms there - or merge with them". That would be like making a Mac program run on a Windows computer. The odds of these other life-forms having compatible DNA is very, very tiny indeed. The only chance that there might be compatibility would be if our life came from their planet or their life from ours (See panspermia) - which means that they aren't really "alien" life at all - just a distant offshoot of our evolutionary tree.
- But that doesn't mean that there can't be some really freakishly weird aliens out there. What if a species of fairly conventional aliens - similar to humans, perhaps - learned how to do really serious nanotechnology - or to produce artificially intelligent self-reproducing robots. Suppose then that over eons, the original species went extinct. That would allow either a race of intelligent robots - or an entire ecology of nano-mechanical "bacteria" to appear and colonize their planet. They might eventually learn to evolve and forget who made them. They might well not depend on carbon, water and reasonable temperatures. We would see them as exceedingly alien creatures!
- I'm sure that even stranger things are possible - and as I said in a previous thread, there is life as we know it, life as we don't know it (but could imagine) and life as we can't even imagine. (Who said that? I can't find it in Wikiquotes.) By definition we can't guess at what might be in the third category. SteveBaker (talk) 02:26, 23 January 2010 (UTC)
Preventing deterioration of rubber-made articles
Rubber tires (and generally things made of rubber) will deteriorate over time, even when they are not used. Are there ways to prevent rubber from deteriorating while in storage? --173.49.12.84 (talk) 21:17, 22 January 2010 (UTC)
- Typically, avoid contact with direct strong light, extreme temperatures, and harsh chemicals, fumes, or abrasives. If you're seeking to preserve the rubber for very long periods of time, you could immerse it in some kind of nonreactive oil bath. To some extent, over long time scales, the rubber will self-react, breaking down its polymer chemical structure, resulting in a more brittle, less elastic material. Nimur (talk) 21:27, 22 January 2010 (UTC)
- I'd go with a nitrogen (or argon) atmosphere, rather than an oil bath. IIRC, some of the components in rubber are oil-soluable. --Carnildo (talk) 01:16, 23 January 2010 (UTC)
- Fellow antique car restorers often recommend Johnson's baby oil. It helps to keep rubber supple by preventing it from drying out - and can even go some way to restoring moderately dried out rubber parts as it's absorbed by the rubber and makes it swell, restoring flexibility. I didn't believe this - but I tried it on some of the rubber and plastic parts of my 1963 Mini - and it works! The stuff can dissolve rubber though - so use a small amount and test it on a bit that doesn't matter too much. Care is needed. SteveBaker (talk)
Surface temperature of planets and moons
Surface temperature can be measured by the infra-red coming off things. What are the surface temperatures of the various planets (solar and extra-solar) and moons. Are they all the same? 84.13.39.208 (talk) 23:54, 22 January 2010 (UTC)
- Venus is way much hotter than the moon or Mars get on their worst days. It's like an acidic steam bath. Or as Carl Sagan once called it, "a thoroughly nasty place". ←Baseball Bugs What's up, Doc? carrots→ 23:59, 22 January 2010 (UTC)
- Go to List of Solar System objects, click on all the links and look in the infobox at the top right. The surface temperatures should all be there. --Tango (talk) 00:03, 23 January 2010 (UTC)
- They are. Venus averages 735 Kelvin, which is pretty freakin' toasty. Mars, in contrast, never gets above freezing. ←Baseball Bugs What's up, Doc? carrots→ 00:19, 23 January 2010 (UTC)
- Bugs, in the name of all that is holy, would you please make an effort to provide a reference each time you post on the Reference Desk? If you had troubled to link to Climate of Mars in this case, for example, you'd see that the estimated high temperature on Mars is 27 degrees Celsius, which is well above freezing, and you would not have posted your inaccurate offhand opinion. Comet Tuttle (talk) 01:40, 23 January 2010 (UTC)
- Tango already linked to List of Solar System objects, which led to my followup comment. Unless you wanted it twice (which you now have). ←Baseball Bugs What's up, Doc? carrots→ 02:14, 23 January 2010 (UTC)
- Specifically, the Mars page (reachable from List of Solar System objects - so there it is a third time) says the MAX is 268 Kelvin or minus 5 Celsius. By definition, that's below freezing. Now, if the the climate page you linked to doesn't agree, maybe you would want to look into reconciling those pages, or at least making the facts clearer. ←Baseball Bugs What's up, Doc? carrots→ 02:17, 23 January 2010 (UTC)
- Tango already linked to List of Solar System objects, which led to my followup comment. Unless you wanted it twice (which you now have). ←Baseball Bugs What's up, Doc? carrots→ 02:14, 23 January 2010 (UTC)
- Bugs, in the name of all that is holy, would you please make an effort to provide a reference each time you post on the Reference Desk? If you had troubled to link to Climate of Mars in this case, for example, you'd see that the estimated high temperature on Mars is 27 degrees Celsius, which is well above freezing, and you would not have posted your inaccurate offhand opinion. Comet Tuttle (talk) 01:40, 23 January 2010 (UTC)
- Thermal maps have been produced for some extrasolar planets, and it appears some are rather hot while others have plenty of atmospheric circulation going from warm to cold. ~AH1(TCU) 02:07, 23 January 2010 (UTC)












