Social history of viruses
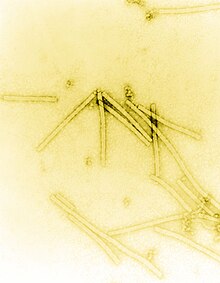
The recorded history of viruses began in the closing years of the 19th century, but their origins date from the time life first appeared on Earth. They infect organisms in all the three domains of life and possibly pre-date the emergence of cells.
Viruses have evolved to become the most successful parasites and have infected plants and animals, including humans, for millions of years. The nature of viruses was not understood until the beginning of the 20th century. Many infectious diseases of plants and animals had been shown to be caused by bacteria and other microorganisms, but no cause could be found for some infections – such as smallpox – that had been recognised for over a thousand years.
Although Louis Pasteur and Edward Jenner developed the first vaccines to protect against viral infections, they did not know that viruses existed. The first evidence of the existence of viruses came from experiments with filters that had pores small enough to retain bacteria. In 1892, Dmitry Ivanovsky used one of these filters to show that sap from a diseased tobacco plant remained infectious to healthy tobacco plants despite having been filtered. Martinus Beijerinck called the filtered, infectious substance a "virus" and this discovery is considered to be the beginning of virology – the study of viruses and viral infections.
In the 20th century many diseases were proved to be caused by viruses. Although scientists' interest in them arose because of the diseases they cause, most viruses are beneficial and they are the most abundant biological entity on Earth. They have driven evolution by transferring genes across species, play important roles in ecosystems, and are essential to life.
Origins
Viruses are ancient and studies at the molecular level have revealed relationships between viruses that infect organisms from the three domains of life and viral proteins that pre-date the divergence of life and thus the last universal common ancestor.[1] This indicates that viruses emerged early in the evolution of life and existed before the modern cells.[2]
There are three classical hypotheses on the origins of viruses: Viruses may have once been small cells that parasitised larger cells. This is called the degeneracy hypothesis,[3][4] or reduction hypothesis.[5] Some viruses may have evolved from bits of DNA or RNA that "escaped" from the genes of a larger organism. This is called the vagrancy hypothesis,[3][6] or the escape hypothesis.[5] The virus-first hypothesis proposes viruses could have evolved from complex molecules of protein and nucleic acid at the same time as cells first appeared on earth.[5]
Historically, none of these hypotheses were fully accepted: The regressive hypothesis did not explain why even the smallest of cellular parasites do not resemble viruses in any way. The escape hypothesis did not explain the complex capsids and other structures on virus particles. The virus-first hypothesis was quickly dismissed because it contravened the definition of viruses, in that they require host cells.[5] However, virologists are beginning to reconsider and re-evaluate all three theories.[7][8]
Evolution
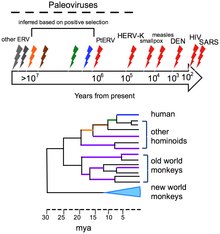
Viruses do not form fossils because they are much smaller than the grains of sedimentary rocks that fossilize plants and animals, therefore the evolution of viruses has had to be traced by other methods. Of these, DNA sequencing, has been the most powerful and has provided unexpected insights.[10] Computers are used to measure viral relationships by comparing their DNA or RNA sequences. As would be expected, viruses of the same genus have much of their sequences in common and more distantly related viruses less in common and this is used to draw phylogenetic trees. The mutation rates for many viruses have been measured, allowing estimates to be made of when species of viruses diverged from common ancestors.[11]
The human genome is a living database of ancient and extinct viruses that once infected hominids and primates. These viruses have left copies of their DNA in the DNA of modern humans, and about 31 different families of viruses, called human endogenous retroviruses, have been discovered.[9] Retroviruses are unusual. Although they are RNA viruses, their reproduction involves a stage where their genes are translated into DNA and inserted into the host cell's DNA.[12] Most of these DNA copies stay in the host cell's genome permanently. A few enter the DNA of the reproductive cells and are passed down through generations of the host's offspring.[13] The last addition to the human germ line is estimated to have occurred around 100,000 to 1,000,000 years ago.[9] The discovery of this ancient, once viral DNA in the human genome has given birth to the science of paleovirology, and although still in its infancy it has already provided insights into the co-evolution of humans and viruses and our development of resistance to them. The study of these "virtual fossils" has become a valuable tool in the study of virus evolution.[9]
Viruses evolve, some quite rapidly. This follows changes in their DNA (or RNA) and the best adapted mutants quickly outnumber their less fit counterparts. In this sense their evolution is Darwinian, just like their host organisms.[14] The way viruses reproduce in their host cells makes them particularly susceptible to the genetic changes that help to drive their evolution.[15] The RNA viruses are especially prone to mutations.[16] These viruses have genes made from RNA not DNA. In host cells there are mechanisms for correcting mistakes when DNA replicates and these kick in whenever cells divide.[16] These important mechanisms prevent potentially lethal mutations from being passed on to offspring. However, these mechanisms do not work for RNA and when an RNA virus replicates in its host cell, changes in their genes are occasionally introduced in error and some of these are lethal. However, one virus particle can produce millions of progeny viruses in just one cycle of replication, so the production of a few "dud" viruses is not a problem. Most mutations are "silent" and do not result in any obvious changes to the progeny viruses. But others confer advantages that increases the fitness of the viruses in the environment. These could be changes to the virus particles that disguise and are not identified by the cells of the immune system or changes that make antiviral drugs less effective. Both of these changes occur with alarming frequency with HIV.[17]
Many viruses can "shuffle" their genes with other viruses when two similar strains infect the same cell. Influenza virus does this and gives rise to a phenomenon called genetic shift, which is often the cause of new and more virulent strains appearing. Other viruses change more slowly as mutations in their genes gradually accumulate over time, and this is called genetic drift.[18]
Through these mechanisms, new viruses are constantly emerging and present a continuing challenge to attempts to control the diseases they cause.[19][20] Most species of viruses are now known to have common ancestors and although the "first virus" hypothesis has yet to gain full acceptance, there is little doubt that the thousands of species of modern viruses have evolved from less numerous ancient species.[21]
Viruses in prehistory
The Neanderthals were a species of hominid that lived in Europe for about 200,000 years but became extinct about 28,000 years ago. The cause of their extinction has not been proven, but an hypothesis published in 2010 suggests that herpes virus infections, which had been brought to Europe from Africa by modern humans (Homo sapiens) might have been responsible.[22]
The Neolithic age, which began in the Middle East around 9500 BC, was a time when humans became farmers. This agricultural revolution embraced the development of monoculture and presented an opportunity for the rapid spread of many plant viruses.[23] The divergence and spread of sobemoviruses – southern bean mosaic virus – date from this time,[24] and the spread of the potyviruses that infect potatoes, and other fruits and vegetables, began about 6,600 years ago.[23]
Over the past 50,000–100,000 years, as humans dispersed throughout the world new infectious diseases, including those caused by viruses, emerged.[25] Smallpox, which was one of the most lethal and devastating viral infections, emerged first among agricultural communities in Africa around 10,000 BC.[26] The virus, which only infected humans, probably descended from the poxviruses that infected rodents.[27] It is probable that early humans hunted these rodents and some people became infected by the viruses that they carried. When viruses cross this so called "species barrier" their effects can be severe[28] and humans may have had little natural resistance. At the time, humans lived in small communities and those who succumbed to infection either died or developed immunity. In humans, this acquired immunity is only passed down to offspring temporarily through breast milk and the antibodies that cross the placenta from the mother's blood to the unborn child's. So, sporadic outbreaks probably occurred in each generation. Around 9000 BC, when many people began to settle on the fertile flood plains of the River Nile, the population became dense enough for the virus to maintain a constant presence owing to the high concentration of susceptible people.[29]
Other, more ancient, viruses are less of a threat. Humans have lived with herpes virus infections since our species first came into being. The virus passed to us from other mammals over 80 million years ago.[30] Humans have developed a tolerance to these viruses, and most of us are infected with a least one species of them without being aware of it. Records of these milder virus infections are understandably rare. But, there is no reason to doubt that early hominids suffered from colds, 'flu and diarrhoea caused by viruses just as humans do today. It is the younger viruses that cause epidemics and pandemics – and it is these that history records.[30]
Viruses in antiquity

Among the earliest records of a viral infection is an Egyptian stele thought to depict an Egyptian priest from the 18th Dynasty (1580–1350 BC) with a foot drop deformity characteristic of a poliovirus infection.[31] The mummy of Siptah – a ruler during the 19th Dynasty – also shows signs of poliomyelitis and that of Ramesses V, and other Egyptian mummies buried over 3000 years ago, show evidence of smallpox.[32]
Measles is an old disease but it was not until the 10th century, that the Persian physician Muhammad ibn Zakariya al-Razi (865–925) (known as "Rhazes") first identified it.[33] Rhazes used the Arabic name "hasbah" for measles, but it has had many other names including "rubeola" from the Latin word rubeus, which means red, and "morbilli", which means "small plague".[34] Measles probably became a disease of humans in the Middle East around 4000 BC. Measles virus is closely related to canine distemper virus and measles first appeared in humans when dogs were first domesticated. One measles infection confers life-long immunity, so the virus requires a high population density to become endemic and so probably did not occur in the Neolithic.[33] Following the emergence of the virus in the Middle East it reached India by 2500 BC.[35] Measles was so common in children at the time that it was not recognised as a disease. In Egyptian hieroglyphs it was described as a normal stage of human development.[36] The Americas and Australia remained free of measles and smallpox until the arrival of European colonists between the fifteenth and eighteenth centuries.[25]
One of the earliest descriptions of a virus-infected plant can be found in a poem written by the Japanese Empress Kōken (718 –770) in which she describes a plant in Summer with yellowing leaves. The plant, later identified as Eupatorium lindleyanum, is often infected with Tomato yellow leaf curl virus.[37]
Middle Ages

The Middle Ages was a time of plagues and pestilences. The rapidly growing population of Europe and the rising concentrations of people in its towns and cites became a fertile ground for many infectious and contagious diseases. The Black Death – a bacterial infection – is probably the most notorious of these.[38] Apart from smallpox and influenza, documented outbreaks of infections now known to be caused by viruses are rare. Rabies, a disease that had been recognised for over 4000 years,[39] was rife in Europe,[40] and continued to be until the development of a vaccine by Louis Pasteur in 1886.[41] The average life-expectancy in the Middle Ages was 35 years and 60% of children died before the age of sixteen, many of them during the first six years of life. Among the plethora of diseases common at the time were influenza, measles and smallpox.[42]
Measles was endemic throughout the highly populated countries of Europe, North Africa and the Middle East. In England the disease, then called "mezils", was first described in the 13th century and was probably one of the 49 plagues that occurred between 526 and 1087.[35]
Early modern period

In 1546 Girolamo Fracastoro (1478–1553) wrote a classic description of measles and thought that the disease was caused by "seeds" (seminaria) that were spread from person to person. In 1670, an epidemic hit London, which was recorded by Thomas Sydenham (1624–1689) who thought it to be caused by toxic vapours emanating from the earth.[35] Sydenham had been a soldier and fought for the Parliamentarians during the English Civil War. As a physician, he was a skilled observer and kept meticulous records.[43]
A short time after Henry Tudor's victory at the Battle of Bosworth on 22 August 1485, his army suddenly went down with "the English sweat", which contemporary observers described as something new.[44] It was probably influenza, but records from the time when medicine was not a science can be unreliable.[45] The language used to describe diseases in these sources is often vague and colloquial. We read of "plagues of mice", "changes in the direction of the wind" and the "influence of comets". This makes retrospective diagnosis difficult. As medicine became a science, the descriptions of disease became less vague and the records became more reliable.
References to influenza infections date from the late 15th and early 16th centuries,[46] but infections almost certainly occurred long before this time.[47] The first that was reliably recorded began in Malta in July 1580, and swept across Europe, Africa and Asia.[48] More than a century passed before there occurred the three pandemics of the eighteenth century, and the one that took place during 1781–2 was probably the most devastating in history.[49]
Along with measles and influenza, smallpox was brought to the Americas by the Spanish.[50] In 1519, an epidemic of smallpox broke out in the Aztec capital Tenochtitlan, which was started by a member of the army of Pánfilo de Narváez (1478–1528). When the Spanish finally entered the capital in the summer of 1521, they saw it strewn with the bodies of smallpox victims.[51] The epidemic, and those that followed during 1545–1548 and 1576–1581, eventually killed more than half of the native population.[52] History repeated itself and many Native American populations were devastated later by the inadvertent spread of smallpox, measles and influenza.[25] It is not known exactly how many were killed, but the damage done by this disease significantly aided European attempts to displace and conquer the native population.[53]

By the 18th century, smallpox was endemic in Europe. There were five epidemics in London between 1719 and 1746, and large outbreaks occurred in other major European cities. By the end of the century, around 400,000 Europeans were dying from the disease each year.[54] The disease reached South Africa in 1713, having been carried by ships from India and in 1789 the disease struck Australia.[54]
Yellow fever is an often lethal disease that is caused by a flavivirus. The virus is transmitted to humans by mosquitoes (Aedes aeypti) and first appeared over 3,000 years ago.[55] In 1647, the first recorded epidemic occurred on Barbados and was called "Barbados distemper" by John Winthrop (1588–1649), who was the Governor of the island at the time. He passed quarantine laws to protect the people – the first ever such laws in north America.[56] More epidemics of the disease occurred in the north Americas in the 17th, 18th and 19th centuries.[57]
Pioneers

Despite his other successes, Louis Pasteur (1822–1895) was unable to find a causative agent for rabies and speculated about a pathogen too small to be detected using a microscope.[58] In 1884, the French microbiologist Charles Chamberland (1851–1931) invented a filter – known today as the Chamberland filter – that had pores smaller than bacteria. Thus, he could pass a solution containing bacteria through the filter and completely remove them from the solution.[59]
In 1892, the Russian biologist Dmitry Ivanovsky (1864–1920) used this filter to study what is now known as the tobacco mosaic virus. His experiments showed that crushed leaf extracts from infected tobacco plants remain infectious after filtration. Ivanovsky suggested the infection might be caused by a toxin produced by bacteria, but did not pursue the idea.[60]
In 1898, the Dutch microbiologist Martinus Beijerinck (1851–1931) repeated the experiments and became convinced that the filtered solution contained a new form of infectious agent.[61] He observed that the agent multiplied only in cells that were dividing and he called it a contagium vivum fluidum (soluble living germ) and re-introduced the word virus.[60] Beijerinck maintained that viruses were liquid in nature, a theory later discredited by the American biochemist and virologist Wendell Meredith Stanley (1904–1971), who proved they were particles.[60] In the same year Friedrich Loeffler (1852–1915) and Paul Frosch (1860–1928) passed the first animal virus through a similar filter and discovered the cause of foot-and-mouth disease.[62]
In 1881, Carlos Finlay (1833–1915), a Cuban physician, first suggested that mosquitoes were carrying the cause of yellow fever,[63] a theory that was proved in 1900 by Walter Reed (1851–1902). During 1901 and 1902, William Crawford Gorgas (1854–1920) organised the destruction of the mosquitoes' breeding habitats in Cuba, which dramatically reduced the prevalence of the disease.[64] Gorgas later organised the elimination of the mosquitoes from Panama, which allowed the Panama Canal to be opened in 1914.[65] The virus was finally isolated by Max Theiler (1899–1972) in 1932 who went on to develop a successful vaccine.[66]
By 1928 enough was known about viruses to enable Thomas Milton Rivers (1888–1962) to write the first book about all known viruses and his Filtrable Viruses was published in 1928. Rivers, who survived typhoid fever at the age of twelve, went on to have a distinguished career in virology. He was born in Jonebro, Georgia USA, was awarded a BA degree from Emory College in 1909 and graduated in medicine at John Hopkins University in 1915. In 1926, he was invited to speak at a meeting organised by the Society of American Bacteriology where he said for the first time, "Viruses appear to be obligate parasites in the sense that their reproduction is dependent on living cells."[67]
That viruses were particles was not considered unnatural and fitted in nicely with the germ theory. It is assumed that Dr. J. Buist of Edinburgh was the first person to see virus particles in 1886, when he reported seeing "micrococci" in vaccine lymph. But he had probably seen clumps of vaccinia virus.[68] In the years that followed, as optical microscopes were improved "inclusion bodies" were seen in many virus-infected cells, but these aggregates of virus particles were still too small to reveal any detailed structure. It was not until the invention of the electron microscope in 1931 by the German engineers Ernst Ruska (1906–1988) and Max Knoll (1887–1969),[69] that virus particles, especially bacteriophages, were shown to have a complex structure. The sizes of viruses determined using this new microscope fitted in well with those estimated by filtration experiments. Viruses were expected to be small, but the range of sizes came as a surprise. Some were only a little smaller than the smallest known bacteria, and the smaller viruses were of similar sizes to complex organic molecules.[70]
In 1935, Wendell Stanley examined the tobacco mosaic virus and found it was mostly made of protein.[71] In 1939, Stanley and Max Lauffer (1914) separated the virus into protein and RNA parts.[72] The discovery of RNA in the particles was important because in 1928, Fred Griffith (c.1879–1941) provided the first evidence that its "cousin", DNA, formed genes.[73]
In Pasteur's day, and for many years after his death the word "virus" was used to describe any cause of infectious disease. Painstaking work, by many bacteriologists, soon discovered the cause of numerous infections. However, some infections remained, many of them horrendous, but for which no bacterial cause could be found. These agents were invisible and could only be grown in living animals. The discovery of viruses was the key that unlocked the door that withheld the secrets of the cause of these mysterious infections. And, although Koch's postulates could not be fulfilled for many of these infections, this did not stop the pioneer virologists from looking for viruses in infections for which no other cause could be found.[74]
Bacteriophages

Discovery
Bacteriophages are the viruses that infect and reproduce in bacteria. They were discovered in the early 20th century, by the English bacteriologist Frederick Twort (1877–1950).[75] But before this time, in 1896, the bacteriologist Ernest Hanbury Hankin (1865–1939) reported that something in the waters of the River Ganges could kill Vibrio cholera – the cause of cholera. Whatever it was in the water could be passed through filters that remove bacteria but was destroyed by boiling.[76] Twort discovered the action of bacteriophages on staphylococci bacteria. He noticed that when grown on nutrient agar some colonies of the bacteria became watery or "glassy". He collected some of these watery colonies and passed them through a Chamberland filter to remove the bacteria and discovered that when the filtrate was added to fresh cultures of bacteria, they in turn became watery.[75] He proposed that the agent might be "an amoeba, an ultramicroscopic virus, a living protoplasm, or an enzyme with the power of growth".[76]
Félix d'Herelle (1873–1949) was a mainly self-taught French-Canadian microbiologist. In 1917 he discovered that "an invisible antagonist", when added to bacteria on agar, would produce areas of dead bacteria.[75] The antagonist, now known to be a bacteriophage could pass through a Chamberland filter. He accurately diluted a suspension of these viruses and discovered that the highest dilutions (lowest virus concentrations), rather than killing all the bacteria, formed discrete areas of dead organisms. Counting these areas and multiplying by the dilution factor allowed him to calculate the number of viruses in the original suspension.[77] He realised that he had discovered a new form of virus and later coined the term "bacteriophage".[78][79] Between 1918 and 1921 d'Herelle discovered different types of bacteriophages that could infect several other species of bacteria including Vibrio cholera.[80] Bacteriophages were heralded as a potential treatment for diseases such as typhoid and cholera, but their promise was forgotten with the development of penicillin.[81] Since the early 1970s, bacteria have continued to develop resistance to antibiotics such as penicillin, and this has led to a renewed interest in the use of bacteriophages to treat serious infections.[82]
Early research 1920–1940
D'Herelle travelled widely to promote the use of bacteriophages in the treatment of bacterial infections. In 1928, he became professor of biology at Yale and founded several research institutes.[83] He was convinced that bacteriophages were viruses despite opposition from established bacteriologists such as the Nobel Prize winner Jules Bordet (1870–1961). Bordet argued that bacteriophages were not viruses but just enzymes released from "lysogenic" bacteria. He said "the invisible world of d'Herelle does not exist".[84] But in the 1930s, the proof that bacteriophages were viruses was provided by Christopher Andrews (1896–1988) and others. They showed that these viruses differed in size and in their chemical and serological properties. In 1940, the first electron micrograph image of a bacteriophage was published and this silenced sceptics who had argued that bacteriophages were relatively simple enzymes and not viruses.[85] Numerous other types of bacteriophages were quickly discovered and were shown to infect bacteria wherever they are found. But this early research was interrupted by World War II. Even d'Herelle was silenced. Despite his Canadian citizenship, he was interned by the Vichy Government until the end of the war.[86]
Modern era
Knowledge of bacteriophages increased in the 1940s following the formation of the Phage Group by scientists throughout the US. Among the members were Max Delbrück (1906–1981) who founded a course on bacteriophages at Cold Spring Harbor Laboratory.[82] He showed that bacterial resistance to infection by bacteriophages was caused by random genetic mutations and not by adaptation.[87] Other key members of the Phage Group included Salvador Luria (1912–1991) and Alfred Hershey (1908–1997). During the 1950s, Hershey and Chase made important discoveries on the replication of DNA during their studies on a bacteriophage called T2. Together with Delbruck they were jointly awarded the 1969 Nobel Prize in Physiology or Medicine "for their discoveries concerning the replication mechanism and the genetic structure of viruses".[88] Since then, the study of bacteriophages has provided insights into the switching on and off of genes, and a useful mechanism for introducing foreign genes into bacteria and many other fundamental mechanisms of molecular biology.[89]
Plant viruses

Many paintings can be found in the museums of Europe that depict tulips with attractive coloured stripes. Most of these, such as the still life studies of Johannes Bosschaert, were painted in the seventeenth century. These flowers were particularly popular and became sought after by those who could afford them. It was not known at the time that these attractive – and very expensive – stripes were caused by a virus that was accidentally transferred by humans to tulips from jasmine.[90]
Before the Irish Great Famine of 1845–1852, the commonest cause of disease in potatoes was not the mould that causes blight but a virus. The disease, called "curl" is caused by Potato leafroll virus and it was widespread in England in the 1770s where it destroyed 75% of the potato crop. At the time, the Irish potato crop remained relatively unscathed.[91]
In 1882, Adolf Mayer (1843–1942) described a condition of tobacco plants, which he called "mosaic disease" ("mozaïkziekte"). The diseased plants had variegated leaves that were mottled.[92] He excluded the possibility of a fungal infection and could not detect any bacterium and speculated that a "soluble, enzyme-like infectious principle was involved".[93] He did not pursue his idea any further, and it was the filtration experiments of Ivanovsky and Beijerinck that suggested the cause was a previously unrecognised infectious agent. After tobacco mosaic was recognized as a virus disease, virus infections of many other plants were discovered.[93]

The importance of tobacco mosaic virus in the history of viruses cannot be overstated. It was the first virus to be discovered, and the first to be crystallised and its structure shown in detail. The first X-ray diffraction pictures of the crystallised virus were obtained by Bernal and Fankuchen in 1941. On the basis of her pictures, Rosalind Franklin discovered the full DNA structure of the virus in 1955.[94] In the same year, Heinz Fraenkel-Conrat and Robley Williams showed that purified tobacco mosaic virus RNA and its coat protein can assemble by themselves to form functional viruses, suggesting that this simple mechanism was probably the means through which viruses were created within their host cells.[95]
By 1935 many plant diseases were thought to be caused by viruses. In 1922, John Kunkel Small (1869–1938) discovered that insects could act as vectors and transmit virus to plants. In the following decade many diseases of plants were shown to be caused by viruses that were carried by insects and in 1939, Francis Holmes, a pioneer in plant virology,[96] described 129 viruses that caused disease of plants.[97] Modern, intensive agriculture provides a rich environment for many plant viruses. In 1948, in Kansas, US, 7% of the wheat crop was destroyed by wheat streak mosaic virus. The virus was spread by mites called Aceria tulipae.[98]
In 1970, the Russian plant virologist Joseph Atabekov discovered that many plant viruses only infect a single species of host plant.[96] The International Committee on Taxonomy of Viruses now recognises over 900 plant viruses.[99]
Viruses of animals
Animals, both wild and domesticated, are susceptible to numerous viral infections. Such disease have been recognised since antiquity. Around 10,000 years ago, the humans that inhabited the lands around the Mediterranean basin began to domesticate wild animals. Pigs, cattle, goats, sheep, horses, camels, cats and dogs were all kept and bred in captivity.[100] These animals would have brought their viruses with them. The transmission of viruses from animals to humans can occur, but these zoonotic infections are rare and subsequent human-to-human transmission is even rarer. Most viruses are species-specific and would have posed no particular threat to humans. But as humans became more dependent on domesticated animals, any outbreaks of disease among their livestock – in animals these are called epizootics – could have devastating consequences.
In modern times epizootics in livestock caused by viruses has had serious consequences. Bluetongue disease, a disease caused by an orbivirus broke out in sheep in France in 2007.[101] Before then, the disease had been mainly confined to the Americas, Africa, southern Asia and northern Australia, but it is now an emerging disease around the Mediterranean.[102]
Foot-and-mouth disease is a highly contagious infection caused by an aphthovirus and is classified in the same family as poliovirus. The virus has infected animals, mainly ungulates, in Africa since ancient times and was probably brought to the Americas in the 19th century by imported livestock.[103] Foot-and-mouth disease is rarely fatal, but the economic losses incurred by outbreaks in sheep and cattle herds can be high.[104] The last occurrence of the disease in the US was in 1929, but as recently as 2001, several large outbreaks occurred throughout the UK and thousands of animals were killed and burnt.[105]
Influenza is mainly a disease of pigs and birds, but has probably infected humans since antiquity.[106] The virus can cause mild to severe infections in wild and domesticated animals.[107] Many species of wild birds migrate and this has spread influenza across the continents throughout the ages. During this time the virus evolved into numerous strains – and continues to do so – posing an ever present threat on humans and their livestock.[108]
Viruses of humans
Discovery of vaccination

Humanity owes a debt to Lady Mary Wortley Montagu (1689–1762). She was an aristocrat, a writer and the wife of a Member of Parliament. In 1716, her husband, Edward Wortley Montagu was appointed British Ambassador in Istanbul. She followed him there and two weeks after her arrival discovered the local practice of protection against smallpox by variolation – the injection of pus from smallpox victims into the skin.[109] Her younger brother had died of smallpox, and she too had had the disease but survived. Determined to spare her five-year-old son Edward from similar suffering, she ordered the embassy surgeon, Charles Maitland to variolate him. On her return to London, she asked Maitland to variolate her four-year-old daughter in the presence of the King's physicians.[110] Later, Montagu persuaded the Prince and Princess of Wales to sponsor a public demonstration of the procedure. Six men who had been condemned to death and were awaiting execution at Newgate Prison were offered a full pardon for serving as the subjects of the public experiment. They accepted, and in 1721 were variolated. All the prisoners recovered from the procedure and to test its protective effect one of them, a nineteen-year-old woman, was ordered to sleep in the same bed as a ten-year old smallpox victim for six weeks. She did not contract the disease.[111] The experiment was repeated on six orphan children who survived the ordeal and by 1722, even King George I's grandchildren had been inoculated. But the practice was not entirely safe and caused many deaths. Although the number of lives saved far outweighed those lost, the practise was not widely adopted.[112]

Edward Jenner (1749–1823), a British rural physician, was variolated as a boy. He had suffered greatly from the ordeal but survived fully protected from smallpox. Jenner knew of a local belief that those who had suffered from a relatively mild infection called cowpox, which was common at the time in dairy workers, were immune to smallpox. Although probably not the first to do so, he decided to test the "theory". On May 14, 1796 he selected "a healthy boy, about eight years old for the purpose of inoculation for the Cow Pox".[113] The boy was James Phipps (1788–1853) who survived the experiment and suffered only a mild fever. On July 1, 1796, Jenner took some "smallpox matter" (probably infected pus) and repeatedly inoculated Phipp's arms with it. Fortunately, Phipps survived, and was later inoculated with smallpox over twenty times without succumbing to the disease. Vaccination – the word is derived from the Latin vacca meaning "cow" – had been invented.[114]
Louis Pasteur and rabies

Rabies is an often fatal disease caused by the infection of mammals with rabies virus. Today it is mainly a disease that affects wild mammals such as foxes and bats, but it is one of the oldest known virus diseases: rabies is a Sanskrit word (rabhas) that dates from 3000 BC,[41] which means "madness" or "rage"[36] and the disease has been known for over 4000 years.[115] The ancient Greeks called it "lyssa" or "lytta" also meaning madness.[36] Although not a cause of epidemics, the infection was greatly feared because of its horrendous symptoms that include insanity, hydrophobia and death.[115] The disease had been known since antiquity and references to rabies can be found in the Laws of Eshnunna, which date from 2300 BC. Aristotle (384–322 BC) wrote one of earliest undisputed descriptions of the disease and how it was passed to humans. Celsus in the first century AD first recorded the symptom called hydrophobia and suggested that the saliva of infected animals and humans contained a slime or poison – to describe this he used the word "virus".[115]
In France, in the time of Louis Pasteur, there were only a few hundred infections in humans each year but cures were desperately sought. Aware of the extreme danger, Pasteur worked on what he knew would be a challenge and he began to look for the "microbe" in the saliva of mad dogs.[116]
A member of Pasteur's team, Emile Roux (1853–1933), studied how long the spinal cords of dogs that had died from the disease remained infectious. Roux invented an ingenious glass bottle in which he hung the spinal cords to dry them. Pasteur was impressed with Roux's invention and ordered more bottles to be made. Pasteur never credited Roux for his invention and Roux considered his idea stolen. Roux never worked on rabies again. After fourteen days of drying, Pasteur showed that when the dried spinal cords were crushed and injected into healthy dogs they did not become infected. He repeated the experiment several times on the same dog with tissue that had been dried for fewer and fewer days, until the dog survived after injections of fresh rabies infected spinal tissue. Pasteur had immunised the dog against rabies as he later did with 50 more.[117]
Although he had little idea how his method worked, he went on to test it on a boy. Joseph Meister (1876–1940) was brought to Pasteur by his mother on July 6, 1885. He was covered in bites, having been set upon by a mad dog. A brick layer had defended the boy from the dog with an iron bar, but only after the salivating dog had bitten the boy fourteen times. Meister's mother begged Pasteur to help her son. Pasteur was a scientist, not a physician, and he was well aware of the consequences to him if things were to go wrong. Pasteur decided to help the boy and injected him with increasingly virulent rabid rabbit spinal cord over the following ten days.[118] Later Pasteur wrote, "as the death of this child appeared inevitable, I decided, not without deep and severe unease...to try out on Joseph Meister the procedure, which had consistently worked on dogs.[119]

The date set for the final inoculation using undried tissue was July 16. Pasteur's assistant Grancher was given the task, but accidentally jabbed himself with the infected needle, and thus became the second guinea pig to receive Pasteur's new treatment. Later in an argument about the ethics of what they had done, Pasteur's whole team, including Pasteur, clandestinely inoculated one another. Although still angry with Pasteur, Roux discovered what was going on and, as he was the only qualified physician on the team, took charge of the dangerous situation. It was too late for the men to stop. Fortunately, they all lived to tell the tale and Meister returned home with his mother on July 27. But the full truth was kept a secret for many years.[120] Pasteur successfully treated a second boy in October of the same year; Jean-Baptiste Jupille (1869–1923) was a fifteen-year-old shepherd boy who had been severely bitten as he tried to protect other children from a rabid dog.[121] Pasteur's method of treatment subsequently remained in use for over fifty years.[122]
Not much was known about the cause of the disease until in 1903 when Adelchi Negri (1876–1912) first saw microscopic lesions in the brains of rabid animals now called Negri bodies.[123] He wrongly thought they were protozoan parasites. However Paul Remlinger (1871–1964) soon showed by filtration experiments that they were much smaller than protozoa and even smaller than bacteria. Thirty years later, Negri bodies were shown to be accumulations of particles 100–150 nanometres long, now known to be the size of rhabdovirus particles – the virus that causes rabies.[115]
20th century
The discovery of viruses, and the control and treatment of the diseases they cause, is one of the great achievements of science in the 20th century.[124] By the end of the 19th century, viruses were defined in terms of their infectivity, their ability to be filtered, and their requirement for living hosts. Up until this time, viruses had only been grown in plants and animals, but in 1906, Ross Granville Harrison (1870–1959) invented a method for growing tissue in lymph,[125] and, in 1913, E Steinhardt, C Israeli, and RA Lambert used this method to grow vaccinia virus in fragments of guinea pig corneal tissue.[126] In 1928, HB and MC Maitland grew vaccinia virus in suspensions of minced hens' kidneys.[127] Their method was not widely adopted until the 1950s, when poliovirus was grown on a large scale for vaccine production.[128]
Yellow fever

In 1905, the last major epidemic of yellow fever occurred in the US.[57] During the building of the Panama Canal thousands of workers succumbed and died from the disease.[129] Yellow fever originated in Africa and the virus was brought to the Americas on cargo ships, which were harbouring the Aedes aegypti that carries the virus. The first recorded epidemic in Africa occurred in Ghana, west Africa, in 1926.[130] In the 1930s, re-emerged in Brazil and Fred Soper, an American epidemiologist (1893–1977) discovered the importance of the sylvatic cycle of infection in non-human hosts and that humans were a "dead end" that broke this cycle.[131] Yellow fever is not the only virus that is carried by insects. In the 1930s apart from yellow fever, only three of these arboviruses were known, dengue and Pappataci fever.[33] Now, over 100 of these viruses are known to cause human diseases.[132] These viruses are diverse and the term "arbovirus", (which was derived from "arthropod borne virus") is no longer used in formal taxonomy because many different species of virus are known to be spread by blood-sucking insects and those that feed on the sap of plants.[133] The yellow fever vaccine is one of the most successful ever developed,[134] but epidemics continue to occur. In 1986–91 epidemics occurred in West Africa and over 20,000 people were infected, 4,000 of whom died.[135] Other arboviruses continue to present problems. West Nile virus arrived in New York in 1999,[136] and, as of 2010, dengue is the most prevalent arbovirus and increasingly virulent strains of the virus have spread across Asia and the Americas.[137]
Influenza

Although the influenza virus that caused the 1918–1919 influenza pandemic was not discovered until the 1930s, the descriptions of the disease and subsequent research has proved it was to blame.[138] The pandemic killed 40–50 million people in less than a year,[139] but the proof that it was caused by a virus was not obtained until 1933.[140] Influenza viruses are relatively large and are difficult to pass through a Chamberland filter and this led many scientists, including the eminent German bacteriologist Richard Pfeiffer (1858–1945), to conclude that the cause was a bacterium.[141] A major breakthrough came in 1931, when the American pathologist Ernest William Goodpasture grew influenza and several other viruses in fertilised chickens' eggs.[142]
Poliomyelitis
In 1949, John F. Enders (1897–1985) Thomas Weller (1915–2008), and Frederick Robbins (1916–2003) grew polio virus for the first time in cultured human embryo cells, the first virus to be grown without using solid animal tissue or eggs. Infections by poliovirus most often cause the mildest of symptoms. This was not known until the virus was isolated in cultured cells and many people were shown to have had mild infections that did not lead to poliomyelitis. But, unlike other viral infections, the incidence of polio – the rarer severe form of the infection – increased in the 20th century and reached a peak around 1952. The invention of a cell culture system for growing the virus enabled Jonas Salk (1914–1995) to make an effective polio vaccine.[143]
Epstein-Barr virus
Denis Parsons Burkitt (1911–1993) was born in Enniskillen, County Fermanagh, Ireland. He was the first to describe a type of cancer that now bears his name Burkitt's lymphoma. This type of cancer was endemic in equatorial Africa and was the commonest malignancy of children in the early 1960s.[144] In an attempt to find a cause for the cancer, Burkitt sent cells from the tumour to Anthony Epstein (b. 1921) a British virologist, who along with Yvonne Barr and Bert Achong (1928–1996), and after many failures, discovered viruses that resembled herpes virus in the fluid that surrounded the cells. The virus was later shown to be a previously unrecognised herpes virus, which is now called Epstein-Barr virus.[145] Surprisingly, Epstein-Barr virus is a very common but relatively mild infection of Europeans. Why it can cause such a devastating illness in Africans is not fully understood, but reduced immunity to virus caused by malaria might be to blame.[146] Epstein-Barr virus is important in the history of viruses for being the first virus shown to cause cancer.[147]
Smallpox eradication
In 1979, the World Health Organisation declared smallpox eradicated, but it had still managed to kill 300 million people since 1900.[148] The eradication followed years of coordinated disease surveillance and vaccination campaigns throughout the world.[149] The main weapon used was vaccinia virus, which was used as the vaccine. No one seems too sure where vaccinia virus came from,[150] it is not the strain of cowpox that Edward Jenner had used and it is not a weakened form of smallpox,[151] but it was a highly successful vaccine.[152]
The eradication campaign was not without casualties. Most notable was the death of Janet Parker (c.1938–1978) and subsequent suicide of virologist Henry Bedson (1930–1978).[153] Before the September 11 attacks on America in 2001, it was planned to destroy all the remaining stocks of smallpox virus that were kept in laboratories in US and Russia. Had this plan gone ahead, smallpox virus would have been the first one to be made extinct by human intervention.[154]
Origin and emergence of HIV

In 1983 Luc Montagnier (b. 1932) and his team at the Pasteur Institute in France, first isolated the retrovirus now called HIV.[155] This virus is one of the most significant that emerged in the last quarter of the 20th century.[156] As of 2010, over 40 million people have been infected and more than 25 million of them have died from AIDS-related diseases.[157] When, in 1981 a report was published reporting the deaths of five young gay men, no one knew of the full scale of the epidemic and that the virus had been silently emerging over several decades.[158] The viruses – there are two of them – HIV-1 and HIV-2, originated in non-human primates in Africa but HIV-1 rapidly spread across the world.[159] Shortly after the HIV-1 virus was discovered in 1983, it was shown to be closely related to SIV, a retrovirus of monkeys and chimpanzees.[160] There are different strains of HIV-1 and HIV-2 and each evolved independently from primates. How these viruses entered the human population is not fully understood, but during the 1960s and 1970s there were large population shifts from rural to urban areas in Africa.[161] The analysis of the RNA from several strains of HIV-1 and HIV-2 has shown that these viruses evolved from SIV-related viruses early in the 20th century.[162]
Late 20th century

The second half of the 20th century was the golden age of virus discovery and most of the 2,000 recognised species of animal, plant, and bacterial viruses were discovered during these years.[87][163] In 1946, Bovine virus diarrhea was discovered,[164] which is still possibly the commonest pathogen of cattle throughout the world[165] and in 1957, equine arterivirus was discovered.[166] In the 1950s, improvements in virus isolation and detection methods resulted in the discovery of several important human viruses including, Varicella zoster virus,[167] the paramyxoviruses,[168] – which include measles virus,[169] and respiratory syncytial virus[168] – and the rhinoviruses that cause the common cold.[170] In the 1960s more viruses were discovered. In 1963, the hepatitis B virus was discovered by Baruch Blumberg (b. 1925),[171] and in 1965, Howard Temin (1934–1994) described the first retrovirus.[172] Reverse transcriptase, the key enzyme that retroviruses use to translate their RNA into DNA, was first described in 1970, independently by Howard Temin and David Baltimore (b. 1938).[173] This was important to the development of antiviral drugs – a key turning-point in the history of viral infections.[174] New viruses and strains of viruses were discovered in every decade of the second half of the 20th century. These discoveries have continued in the 21st century as new viral diseases such as SARS[175] and nipah virus[176] have emerged. Despite scientists' achievements over the past one hundred years, viruses continue to pose new threats and challenges.[177]
Friendly viruses
Sir Peter Medawar (1915–1987) described a virus as "a piece of bad news wrapped in a protein coat".[178] He might have had his tongue firmly in his cheek, but this summed up the view of most virologists in the mid-20th century. With the exception of the bacteriophages, viruses had a well-deserved reputation for being nothing but the cause of diseases and death. The discovery of the abundance of viruses and their overwhelming presence in many ecosystems has led modern virologists to consider them in a new light.[179]
Viruses are everywhere and it has been estimated that there are 1031 viruses on earth. Most of these are bacteriophages found in the oceans.[180] Microorganisms constitute more than 90% of the biomass in the sea,[181] and it has been estimated that viruses kill approximately 20% of this biomass each day and that there are fifteen times as many viruses in the oceans as there are bacteria and archaea.[181] Viruses are the main agents responsible for the rapid destruction of harmful algal blooms, which often kill other marine life,[181] and help maintain the ecological balance of different species of marine blue-green algae.[182]
The Human Genome Project has revealed the presence of numerous viral DNA sequences scattered throughout the human genome.[183] These sequences make up around 8% of human DNA,[9] and appear to be the remains of ancient retrovirus infections of human ancestors.[184] These pieces of DNA have firmly established themselves in human DNA.[185] Most of this DNA is no longer functional and a few sequences might occasionally cause harm. The remainder, however, seem to be beneficial, having brought with them novel genes that are important to human development.[186]
References
- ^ Forterre P. (2009). Desk Encyclopedia of General Virology. Oxford: Academic Press. p. 25. ISBN 0-12-375146-2.
{{cite book}}: Unknown parameter|editors=ignored (|editor=suggested) (help) - ^ Forterre P. (2009). Desk Encyclopedia of General Virology. Oxford: Academic Press. p. 26. ISBN 0-12-375146-2.
{{cite book}}: Unknown parameter|editors=ignored (|editor=suggested) (help) - ^ a b Leppard, Keith; Nigel Dimmock; Easton, Andrew (2007). Introduction to Modern Virology. Blackwell Publishing Limited. p. 16. ISBN 1-4051-3645-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Sussman, Max; Topley, W. W. C.; Wilson, Graham K.; Collier, L. H.; Balows, Albert (1998). Topley & Wilson's microbiology and microbial infections. London: Arnold. p. 11. ISBN 0-340-66316-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Mahy WJ & Van Regenmortel MHV (eds) (2009). Desk Encyclopedia of General Virology. Oxford: Academic Press. p. 24. ISBN 0-12-375146-2.
{{cite book}}:|author=has generic name (help) - ^ Sussman, Max; Topley, W. W. C.; Wilson, Graham K.; Collier, L. H.; Balows, Albert (1998). Topley & Wilson's microbiology and microbial infections. London: Arnold. pp. 11–12. ISBN 0-340-66316-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Mahy WJ & Van Regenmortel MHV (eds) (2009). Desk Encyclopedia of General Virology. Oxford: Academic Press. p. 28. ISBN 0-12-375146-2.
{{cite book}}:|author=has generic name (help) - ^ Forterre P (2010). "Giant viruses: conflicts in revisiting the virus concept". Intervirology. 53 (5): 362–78. doi:10.1159/000312921. PMID 20551688.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c d e Emerman M, Malik HS (2010). "Paleovirology—modern consequences of ancient viruses". Plos Biology. 8 (2): e1000301. doi:10.1371/journal.pbio.1000301. PMC 2817711. PMID 20161719.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: unflagged free DOI (link) - ^ Mahy BWJ and Van Regenmortel MHV (2009). Desk Encyclopedia of General Virology. Boston: Academic Press. pp. 66–79. ISBN 0-12-375146-2.
- ^ Lam TT, Hon CC, Tang JW (2010). "Use of phylogenetics in the molecular epidemiology and evolutionary studies of viral infections". Critical Reviews in Clinical Laboratory Sciences. 47 (1): 5–49. doi:10.3109/10408361003633318. PMID 20367503.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Bannert N, Kurth R (2006). "The evolutionary dynamics of human endogenous retroviral families". Annual Review of Genomics and Human Genetics. 7: 149–73. doi:10.1146/annurev.genom.7.080505.115700. PMID 16722807.
- ^ Jern P, Coffin JM (2008). "Effects of retroviruses on host genome function". Annual Review of Genetics. 42: 709–32. doi:10.1146/annurev.genet.42.110807.091501. PMID 18694346.
- ^ Leppard, Keith; Nigel Dimmock; Easton, Andrew (2007). Introduction to Modern Virology. Blackwell Publishing Limited. p. 273. ISBN 1-4051-3645-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Leppard, Keith; Nigel Dimmock; Easton, Andrew (2007). Introduction to Modern Virology. Blackwell Publishing Limited. p. 272. ISBN 1-4051-3645-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b Domingo E, Escarmís C, Sevilla N, Moya A, Elena SF, Quer J, Novella IS, Holland JJ (1996). "Basic concepts in RNA virus evolution". The FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 10 (8): 859–64. PMID 8666162.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Boutwell CL, Rolland MM, Herbeck JT, Mullins JI, Allen TM (2010). "Viral evolution and escape during acute HIV-1 infection". The Journal of Infectious Diseases. 202 Suppl 2: S309–14. doi:10.1086/655653. PMC 2945609. PMID 20846038.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Chen J, Deng YM (2009). "Influenza virus antigenic variation, host antibody production and new approach to control epidemics". Virology Journal. 6: 30. doi:10.1186/1743-422X-6-30. PMC 2666653. PMID 19284639.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Fraile A, García-Arenal F (2010). "The coevolution of plants and viruses: resistance and pathogenicity". Advances in Virus Research. 76: 1–32. doi:10.1016/S0065-3527(10)76001-2. PMID 20965070.
- ^ Tang JW, Shetty N, Lam TT, Hon KL (2010). "Emerging, novel, and known influenza virus infections in humans". Infectious Disease Clinics of North America. 24 (3): 603–17. doi:10.1016/j.idc.2010.04.001. PMID 20674794.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Mahy BWJ and Van Regenmortel MHV (2009). Desk Encyclopedia of General Virology. Boston: Academic Press. pp. 70–80 "Evolution of Viruses". ISBN 0-12-375146-2.
- ^ Wolff H, Greenwood AD (2010). "Did viral disease of humans wipe out the Neandertals?". Medical Hypotheses. 75 (1): 99–105. doi:10.1016/j.mehy.2010.01.048. PMID 20172660.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Gibbs AJ, Ohshima K, Phillips MJ, Gibbs MJ (2008). "The prehistory of potyviruses: their initial radiation was during the dawn of agriculture". PLoS ONE. 3 (6): e2523. doi:10.1371/journal.pone.0002523. PMC 2429970. PMID 18575612.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Fargette D, Pinel-Galzi A, Sérémé D, Lacombe S, Hébrard E, Traoré O, Konaté G (2008). "Diversification of rice yellow mottle virus and related viruses spans the history of agriculture from the neolithic to the present". PLoS Pathogens. 4 (8): e1000125. doi:10.1371/journal.ppat.1000125. PMC 2495034. PMID 18704169.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b c McMichael AJ (2004). "Environmental and social influences on emerging infectious diseases: past, present and future". Philos. Trans. R. Soc. Lond., B, Biol. Sci. 359 (1447): 1049–58. doi:10.1098/rstb.2004.1480. PMC 1693387. PMID 15306389.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Teri Shors (2008). Understanding Viruses. Sudbury, Mass: Jones & Bartlett Publishers. p. 27. ISBN 0-7637-2932-9.
- ^ Piskurek O, Okada N (2007). "Poxviruses as possible vectors for horizontal transfer of retroposons from reptiles to mammals". Proceedings of the National Academy of Sciences of the United States of America. 104 (29): 12046–51. doi:10.1073/pnas.0700531104. PMC 1924541. PMID 17623783.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Georges AJ, Matton T, Courbot-Georges MC (2004). "[Monkey-pox, a model of emergent then reemergent disease]". Médecine et Maladies Infectieuses (in French). 34 (1): 12–9. doi:10.1016/j.medmal.2003.09.008. PMID 15617321.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Tucker, Jonathan B. (2002). Scourge: the once and future threat of smallpox. New York: Grove Press. p. 6. ISBN 0-8021-3939-6.
- ^ a b Crawford, Dorothy H. (2000). The invisible enemy: a natural history of viruses. Oxford [Oxfordshire]: Oxford University Press. p. 225. ISBN 0-19-856481-3.
- ^ Teri Shors (2008). Understanding Viruses. Sudbury, Mass: Jones & Bartlett Publishers. p. 13. ISBN 0-7637-2932-9.
- ^ Donadoni, Sergio (1997). The Egyptians. Chicago: University of Chicago Press. p. 292. ISBN 0-226-15556-0.
- ^ a b c Levins, Richard; Wilson, Mary E. (1994). Disease in evolution: global changes and emergence of infectious diseases. New York, N.Y: New York Academy of Sciences. pp. 297–298. ISBN 0-89766-876-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) Cite error: The named reference "isbn0-89766-876-6" was defined multiple times with different content (see the help page). - ^ Dobson, Mary J. (2008). Disease. Englewood Cliffs, N.J: Quercus. pp. 140–141. ISBN 1-84724-399-1.
- ^ a b c Retief F, Cilliers L (2010). "Measles in antiquity and the Middle Ages". South African Medical Journal = Suid-Afrikaanse Tydskrif Vir Geneeskunde. 100 (4): 216–7. PMID 20459960.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c Zuckerman, Arie J. (1987). Principles and practice of clinical virology. New York: Wiley. p. 291. ISBN 0-471-90341-8. Cite error: The named reference "isbn0-471-90341-8" was defined multiple times with different content (see the help page).
- ^ Hull R. (2009). Desk Encyclopedia of General Virology. Oxford: Academic Press. p. 10. ISBN 0-12-375146-2.
In this village, It looks as if frosting continuously,For, the plant I saw In the field of Summer, The color of the leaves were yellowing
{{cite book}}: Unknown parameter|editors=ignored (|editor=suggested) (help) - ^ Gottfried RS (1977). "Population, plague, and the sweating sickness: demographic movements in late fifteenth-century England". The Journal of British Studies. 17: 12–37. doi:10.1086/385710. PMID 11632234.
- ^ Kuzmin I.V. and Rupprecht C.E (2009). Desk Encyclopedia of Human and Medical Virology. Oxford: Academic Press. p. 243. ISBN 0-12-375147-0.
- ^ Van Renynghe de Voxvrie G (1993). "[Flemish psychiatry from the middle ages to the 18th century]". Acta Psychiatrica Belgica (in French). 93 (2): 83–96. PMID 8036936.
- ^ a b Teri Shors (2008). Understanding Viruses. Sudbury, Mass: Jones & Bartlett Publishers. p. 352. ISBN 0-7637-2932-9. Cite error: The named reference "Shors 352" was defined multiple times with different content (see the help page).
- ^ Scott, Robert Pickett (2010). Miracle Cures: Saints, Pilgrimage, and the Healing Powers of Belief. Berkeley: University of California Press. p. 10. ISBN 0-520-26275-1.
- ^ Sloan AW (1987). "Thomas Sydenham, 1624–1689". South African Medical Journal = Suid-Afrikaanse Tydskrif Vir Geneeskunde. 72 (4): 275–8. PMID 3303370.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Quinn, Tom (2008). Flu: A Social History of Influenza. New Holland Publishers (UK) LTD. pp. 40–41. ISBN 1-84537-941-1.
- ^ Quinn, Tom (2008). Flu: A Social History of Influenza. New Holland Publishers (UK) LTD. p. 40. ISBN 1-84537-941-1.
- ^ Quinn, Tom (2008). Flu: A Social History of Influenza. New Holland Publishers (UK) LTD. p. 39. ISBN 1-84537-941-1.
- ^ Quinn, Tom (2008). Flu: A Social History of Influenza. New Holland Publishers (UK) LTD. pp. 39–57. ISBN 1-84537-941-1.
- ^ Quinn, Tom (2008). Flu: A Social History of Influenza. New Holland Publishers (UK) LTD. p. 59. ISBN 1-84537-941-1.
- ^ Quinn, Tom (2008). Flu: A Social History of Influenza. New Holland Publishers (UK) LTD. p. 71. ISBN 1-84537-941-1.
- ^ McMichael AJ (2004). "Environmental and social influences on emerging infectious diseases: past, present and future". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 359 (1447): 1049–58. doi:10.1098/rstb.2004.1480. PMC 1693387. PMID 15306389.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Tucker, Jonathan B. (2002). Scourge: the once and future threat of smallpox. New York: Grove Press. p. 10. ISBN 0-8021-3939-6.
- ^ Berdan, Frances (2005). The Aztecs of central Mexico: an imperial society. Belmont, CA: Thomson Wadsworth. pp. 182–183. ISBN 0-534-62728-5.
- ^
- Ranlet P (2000). "The British, the Indians, and smallpox: what actually happened at Fort Pitt in 1763?". Pennsylvania History. 67 (3): 427–41. PMID 17216901.
- Van Rijn K (2006). ""Lo! The poor Indian!" colonial responses to the 1862–63 smallpox epidemic in British Columbia and Vancouver Island". Canadian Bulletin of Medical History = Bulletin Canadien D'histoire De La Médecine. 23 (2): 541–60. PMID 17214129.
- Patterson KB, Runge T (2002). "Smallpox and the Native American". The American Journal of the Medical Sciences. 323 (4): 216–22. doi:10.1097/00000441-200204000-00009. PMID 12003378.
{{cite journal}}: Unknown parameter|month=ignored (help) - Sessa R, Palagiano C, Scifoni MG, di Pietro M, Del Piano M (1999). "The major epidemic infections: a gift from the Old World to the New?". Panminerva Medica. 41 (1): 78–84. PMID 10230264.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)Bianchine PJ, Russo TA (1992). "The role of epidemic infectious diseases in the discovery of America". Allergy Proceedings : the Official Journal of Regional and State Allergy Societies. 13 (5): 225–32. PMID 1483570. - Hauptman LM (1979). "Smallpox and American Indian; Depopulation in Colonial New York". New York State Journal of Medicine. 79 (12): 1945–9. PMID 390434.
{{cite journal}}: Unknown parameter|month=ignored (help) - Fortuine R (1988). "Smallpox decimates the Tlingit (1787)". Alaska Medicine. 30 (3): 109. PMID 3041871.
- ^ a b Tucker, Jonathan B. (2002). Scourge: the once and future threat of smallpox. New York: Grove Press. pp. 12–13. ISBN 0-8021-3939-6.
- ^ Mahy BWJ and Van Regenmortel (2009). Desk Encyclopedia of Human and Medical Virology. Boston: Academic Press. p. 514. ISBN 0-12-375147-0.
- ^ Dobson, Mary J. (2008). Disease. Englewood Cliffs, N.J: Quercus. pp. 146–147. ISBN 1-84724-399-1.
- ^ a b Patterson KD (1992). "Yellow fever epidemics and mortality in the United States, 1693–1905". Soc Sci Med. 34 (8): 855–65. doi:10.1016/0277-9536(92)90255-O. PMID 1604377.
{{cite journal}}: Unknown parameter|month=ignored (help) Cite error: The named reference "pmid1604377" was defined multiple times with different content (see the help page). - ^ Bordenave G (2003). "Louis Pasteur (1822–1895)". Microbes and Infection / Institut Pasteur. 5 (6): 553–60. PMID 12758285.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Teri Shors (2008). Understanding Viruses. Sudbury, Mass: Jones & Bartlett Publishers. pp. 76–77. ISBN 0-7637-2932-9.
- ^ a b c Sussman, Max; Topley, W. W. C.; Wilson, Graham K.; Collier, L. H.; Balows, Albert (1998). Topley & Wilson's microbiology and microbial infections. London: Arnold. p. 3. ISBN 0-340-66316-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Leppard, Keith; Nigel Dimmock; Easton, Andrew (2007). Introduction to Modern Virology. Blackwell Publishing Limited. pp. 4–5. ISBN 1-4051-3645-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Fenner F. (2009). Mahy B. W. J. and Van Regenmortal M. H. V. (ed.). Desk Encyclopedia of General Virology (1 ed.). Oxford, UK: Academic Press. p. 15. ISBN 0-12-375146-2.
- ^ Chiong MA (1989). "Dr. Carlos Finlay and yellow fever". CMAJ. 141 (11): 1126. PMC 1451274. PMID 2684378.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Litsios S (2001). "William Crawford Gorgas (1854–1920)". Perspectives in Biology and Medicine. 44 (3): 368–78. doi:10.1353/pbm.2001.0051. PMID 11482006.
- ^ Patterson R (1989). "Dr. William Gorgas and his war with the mosquito". CMAJ : Canadian Medical Association Journal = Journal De l'Association Medicale Canadienne. 141 (6): 596–7, 599. PMC 1451363. PMID 2673502.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Frierson JG (2010). "The yellow fever vaccine: a history". The Yale Journal of Biology and Medicine. 83 (2): 77–85. PMC 2892770. PMID 20589188.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Frank L. Horsfall Jnr.(1965) "Thomas Milton Rivers (1888–1962)—A biographical memoir" The National Academy of Sciences Washington D.C. Retrieved 3 December 2010.
- ^ *In 1887, Buist visualised one of the largest, Vaccinia virus, by optical microscopy after staining it. Vaccinia was not known to be a virus at that time. (Buist J.B.(1887) Vaccinia and Variola: a study of their life history Churchill, London)
- ^ From Nobel Lectures, Physics 1981–1990, (1993) Editor-in-Charge Tore Frängsmyr, Editor Gösta Ekspång, World Scientific Publishing Co., Singapore.
- ^ Carr, N. G.; Mahy, B. W. J.; Pattison, J. R.; Kelly, D. P. (1984). The microbe 1984: Thirty-sixth Symposium of the Society for General Microbiology, held at the University of Warwick, April 1984. Cambridge: Published for the Society for General Microbiology [by] Cambridge University Press. p. 4. ISBN 0-521-26056-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Stanley WM, Loring HS (1936). "The isolation of crystalline tobacco mosaic virus protein from diseased tomato plants". Science, 83, p. 85 PMID 17756690
- ^ Stanley WM, Lauffer MA (b. 1939). "Disintegration of tobacco mosaic virus in urea solutions". Science 88, pp. 345–347 PMID 17788438
- ^ Burton E. Tropp (2007). Molecular Biology: Genes to Proteins. Burton E. Tropp. Sudbury, Massachusetts: Jones & Bartlett Publishers. p. 12. ISBN 0-7637-5963-5.
- ^ Carr, N. G.; Mahy, B. W. J.; Pattison, J. R.; Kelly, D. P. (1984). The microbe 1984: Thirty-sixth Symposium of the Society for General Microbiology, held at the University of Warwick, April 1984. Cambridge: Published for the Society for General Microbiology [by] Cambridge University Press. p. 3. ISBN 0-521-26056-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e Teri Shors (2008). Understanding Viruses. Sudbury, Mass: Jones & Bartlett Publishers. p. 589. ISBN 0-7637-2932-9.
- ^ a b Ackermann H-W (2009). Desk Encyclopedia of General Virology. Oxford: Academic Press. p. 3. ISBN 0-12-375146-2.
- ^ a b D'Herelle F (September 2007). "On an invisible microbe antagonistic toward dysenteric bacilli": brief note by Mr. F. D'Herelle, presented by Mr. Roux. 1917. Res. Microbiol. 158(7):553–4. Epub 2007 Jul 28. PMID 17855060
- ^ Ackermann H-W (2009). Desk Encyclopedia of General Virology. Oxford: Academic Press. p. 4. ISBN 0-12-375146-2.
- ^ "The antagonistic microbe can never be cultivated in media in the absence of the dysentery bacillus. It does not attack heat-killed dysentery bacilli, but is cultivated perfectly in a suspension of washed cells in physiological saline. This indicates that the anti dysentery microbe is an obligate bacteriophage". Felix d'Herelle (1917) An invisible microbe that is antagonistic to the dysentery bacillus (1917) Comptes rendus Acad. Sci. Paris Retrieved on 2 December 2010
- ^ Ackermann H-W (2009). Desk Encyclopedia of General Virology. Oxford: Academic Press. p. 4 Table 1. ISBN 0-12-375146-2.
- ^ Ackermann H-W (2009). Desk Encyclopedia of General Virology. Oxford: Academic Press. p. 4. ISBN 0-12-375146-2.
- ^ a b Teri Shors (2008). Understanding Viruses. Sudbury, Mass: Jones & Bartlett Publishers. p. 591. ISBN 0-7637-2932-9. Cite error: The named reference "Shors 591" was defined multiple times with different content (see the help page).
- ^ Teri Shors (2008). Understanding Viruses. Sudbury, Mass: Jones & Bartlett Publishers. p. 590. ISBN 0-7637-2932-9.
- ^ Quoted in: Ackermann H-W (2009). Desk Encyclopedia of General Virology. Oxford: Academic Press. p. 4. ISBN 0-12-375146-2.
- ^ Ackermann H-W (2009). Desk Encyclopedia of General Virology. Oxford: Academic Press. pp. 3–5. ISBN 0-12-375146-2.
- ^ Ackermann H-W (2009). Desk Encyclopedia of General Virology. Oxford: Academic Press. p. 5. ISBN 0-12-375146-2.
- ^ a b Norrby E (2008). "Nobel Prizes and the emerging virus concept". Archives of Virology. 153 (6): 1109–23. doi:10.1007/s00705-008-0088-8. PMID 18446425. Cite error: The named reference "pmid18446425" was defined multiple times with different content (see the help page).
- ^ Nobel Organisation
- ^ Ackermann H-W (2009). Desk Encyclopedia of General Virology. Oxford: Academic Press. pp. 5–10 Table 1. ISBN 0-12-375146-2.
- ^ Hull R. (2009). Desk Encyclopedia of General Virology. Oxford: Academic Press. pp. 10–11. ISBN 0-12-375146-2.
- ^ Zuckerman, Larry (1999). The potato: how the humble spud rescued the western world. San Francisco: North Point Press. p. 21. ISBN 0-86547-578-4.
- ^ Mayer A (1882) Over de moza¨ıkziekte van de tabak: voorloopige mededeeling. Tijdschr Landbouwkunde Groningen 2: 359–364 (In German)
- ^ a b Quoted in: van der Want JP, Dijkstra J (2006). "A history of plant virology". Archives of Virology. 151 (8): 1467–98. doi:10.1007/s00705-006-0782-3. PMID 16732421.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Creager AN, Morgan GJ (2008). "After the double helix: Rosalind Franklin's research on Tobacco mosaic virus". Isis. 99 (2): 239–72. doi:10.1086/588626. PMID 18702397.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Leppard, Keith; Nigel Dimmock; Easton, Andrew (2007). Introduction to Modern Virology. Blackwell Publishing Limited. p. 12. ISBN 1-4051-3645-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b Pennazio S, Roggero P, Conti M (2001). "A history of plant virology. Mendelian genetics and resistance of plants to viruses". New Microbiology. 24 (4): 409–24. PMID 11718380.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Teri Shors (2008). Understanding Viruses. Sudbury, Mass: Jones & Bartlett Publishers. p. 563. ISBN 0-7637-2932-9.
- ^ D. Hansing, C. O. Johnston, L. E. Melchers and H. Fellows (1949) "Kansas Phytopathological Notes: 1948" Transactions of the Kansas Academy of Science (1903-) Vol. 52, No. 3, pp. 363-369 Stable URL Retrieved on 13 December 2010
- ^ Teri Shors (2008). Understanding Viruses. Sudbury, Mass: Jones & Bartlett Publishers. p. 564. ISBN 0-7637-2932-9.
- ^ Zeder MA (2008). "Domestication and early agriculture in the Mediterranean Basin: Origins, diffusion, and impact". Proceedings of the National Academy of Sciences of the United States of America. 105 (33): 11597–604. doi:10.1073/pnas.0801317105. PMC 2575338. PMID 18697943.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Durand B, Zanella G, Biteau-Coroller F, Locatelli C, Baurier F, Simon C, Le Drean E, Delaval J, Prengere E, Beaute V, Guis H (2010). "Anatomy of Bluetongue Virus Serotype 8 Epizootic Wave, France, 2007–2008". Emerging Infectious Diseases. 16 (12): 1861–1868. PMID 21122214.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Mellor PS, Carpenter S, Harrup L, Baylis M, Mertens PP (2008). "Bluetongue in Europe and the Mediterranean Basin: history of occurrence prior to 2006". Preventive Veterinary Medicine. 87 (1–2): 4–20. doi:10.1016/j.prevetmed.2008.06.002. PMID 18619694.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)payment required for DOI - ^ Paton DJ, Sumption KJ, Charleston B (2009). "Options for control of foot-and-mouth disease: knowledge, capability and policy". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 364 (1530): 2657–67. doi:10.1098/rstb.2009.0100. PMC 2865093. PMID 19687036.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Scudamore JM, Trevelyan GM, Tas MV, Varley EM, Hickman GA (2002). "Carcass disposal: lessons from Great Britain following the foot and mouth disease outbreaks of 2001". Rev. – Off. Int. Epizoot. 21 (3): 775–87. PMID 12523714.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Mahy BW (2005). "Introduction and history of foot-and-mouth disease virus". Current Topics in Microbiology and Immunology. 288: 1–8. doi:10.1007/3-540-27109-0_1. PMID 15648172.
- ^ Sussman, Max; Topley, W. W. C.; Wilson, Graham K.; Collier, L. H.; Balows, Albert (1998). Topley & Wilson's microbiology and microbial infections. London: Arnold. p. 386. ISBN 0-340-66316-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Suarez DL (2010). "Avian influenza: our current understanding". Anim Health Res Rev. 11 (1): 19–33. doi:10.1017/S1466252310000095. PMID 20591211.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Feare CJ (2010). "Role of wild birds in the spread of highly pathogenic avian influenza virus H5N1 and implications for global surveillance". Avian Dis. 54 (1 Suppl): 201–12. doi:10.1637/8766-033109-ResNote.1. PMID 20521633.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Tucker, Jonathan B. (2002). Scourge: the once and future threat of smallpox. New York: Grove Press. p. 16. ISBN 0-8021-3939-6.
- ^ Tucker, Jonathan B. (2002). Scourge: the once and future threat of smallpox. New York: Grove Press. pp. 16–17. ISBN 0-8021-3939-6.
- ^ Tucker, Jonathan B. (2002). Scourge: the once and future threat of smallpox. New York: Grove Press. p. 17. ISBN 0-8021-3939-6.
- ^ Reid, Robert (1974). Microbes and men. London: British Broadcasting Corporation. p. 13. ISBN 0-563-12469-5.
- ^ Quoted in: Reid, Robert (1974). Microbes and men. London: British Broadcasting Corporation. p. 18. ISBN 0-563-12469-5.
- ^ Reid, Robert (1974). Microbes and men. London: British Broadcasting Corporation. p. 19. ISBN 0-563-12469-5.
- ^ a b c d Mahy BWJ and Van Regenmortel MHV (2009). Desk Encyclopedia of Human and Medical Virology. Oxford: Academic Press. p. 243. ISBN 0-12-375147-0.
- ^ Reid, Robert (1974). Microbes and men. London: British Broadcasting Corporation. pp. 93–94. ISBN 0-563-12469-5.
- ^ Reid, Robert (1974). Microbes and men. London: British Broadcasting Corporation. p. 96. ISBN 0-563-12469-5.
- ^ Reid, Robert (1974). Microbes and men. London: British Broadcasting Corporation. pp. 97–98. ISBN 0-563-12469-5.
- ^ Dobson, Mary J. (2008). Englewood Cliffs, N.J: Quercus. p. 159. ISBN 1-84724-399-1.
As the death of this child appeared inevitable, I decided, not without deep and severe unease, as one can imagine, to try out on Joseph Meister the procedure, which had consistently worked on dogs.
{{cite book}}: Missing or empty|title=(help) - ^ Reid, Robert (1974). Microbes and men. London: British Broadcasting Corporation. pp. 94–100. ISBN 0-563-12469-5.
- ^ Dobson, Mary J. (2008). Disease. Englewood Cliffs, N.J: Quercus. pp. 159–160. ISBN 1-84724-399-1.
- ^ Dreesen DW (1997). "A global review of rabies vaccines for human use". Vaccine. 15: S2–6. doi:10.1016/S0264-410X(96)00314-3. PMID 9218283.
- ^ Kristensson K, Dastur DK, Manghani DK, Tsiang H, Bentivoglio M (1996). "Rabies: interactions between neurons and viruses. A review of the history of Negri inclusion bodies". Neuropathology and Applied Neurobiology. 22 (3): 179–87. doi:10.1111/j.1365-2990.1996.tb00893.x. PMID 8804019.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Oldstone MBA (2009). Viruses, Plagues, and History: Past, Present and Future. Oxford University Press, USA. pp. 19–40. ISBN 0-19-532731-4.
- ^ J. S. Nicholas, Ross Granville Harrison 1870—1959 A Biographical Memoir, National Academy of Sciences, 1961, Retrieved on December 4, 2010
- ^ Steinhardt, E; Israeli, C; and Lambert, R.A. (1913) "Studies on the cultivation of the virus of vaccinia" J. Inf Dis. 13, 294–300
- ^ Maitland HB, Magrath DI (1957). "The growth in vitro of vaccinia virus in chick embryo chorio-allantoic membrane, minced embryo and cell suspensions". The Journal of Hygiene. 55 (3): 347–60. doi:10.1017/S0022172400037268. PMC 2217967. PMID 13475780.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Sussman, Max; Topley, W. W. C.; Wilson, Graham K.; Collier, L. H.; Balows, Albert (1998). Topley & Wilson's microbiology and microbial infections. London: Arnold. p. 4. ISBN 0-340-66316-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Ziperman HH (1973). "A medical history of the Panama Canal". Surgery, Gynecology & Obstetrics. 137 (1): 104–14. PMID 4576836.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Dobson, Mary J. (2008). Disease. Englewood Cliffs, N.J: Quercus. p. 148. ISBN 1-84724-399-1.
- ^ Ansari MZ, Shope RE (1994). "Epidemiology of arboviral infections". Public Health Reviews. 22 (1–2): 1–26. PMID 7809386.
- ^ Mahy BWJ and Van Regenmortel MHV (2009). Desk Encyclopedia of Human and Medical Virology. Boston: Academic Press. ISBN 0-12-375147-0.
{{cite book}}: Cite has empty unknown parameter:|page24=(help) - ^ Weaver SC (2006). "Evolutionary influences in arboviral disease". Current Topics in Microbiology and Immunology. 299: 285–314. doi:10.1007/3-540-26397-7_10. PMID 16568903.
- ^ Barrett AD, Teuwen DE (2009). "Yellow fever vaccine – how does it work and why do rare cases of serious adverse events take place?". Current Opinion in Immunology. 21 (3): 308–13. doi:10.1016/j.coi.2009.05.018. PMID 19520559.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Cordellier R (1991). "[The epidemiology of yellow fever in Western Africa]". Bulletin of the World Health Organization (in French). 69 (1): 73–84. PMC 2393223. PMID 2054923.
- ^ Reiter P (2010). "West Nile virus in Europe: understanding the present to gauge the future". Euro Surveillance : Bulletin Européen Sur Les Maladies Transmissibles = European Communicable Disease Bulletin. 15 (10): 19508. PMID 20403311.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Ross TM (2010). "Dengue virus". Clinics in Laboratory Medicine. 30 (1): 149–60. doi:10.1016/j.cll.2009.10.007. PMID 20513545.
{{cite journal}}: Unknown parameter|month=ignored (help) Cite error: The named reference "pmid20513545" was defined multiple times with different content (see the help page). - ^ Teri Shors (2008). Understanding Viruses. Sudbury, Mass: Jones & Bartlett Publishers. pp. 238–344. ISBN 0-7637-2932-9.
- ^ Oldstone MBA (2009). Viruses, Plagues, and History: Past, Present and Future. Oxford University Press, USA. p. 306. ISBN 0-19-532731-4.
- ^ Cunha BA (2004). "Influenza: historical aspects of epidemics and pandemics". Infectious Disease Clinics of North America. 18 (1): 141–55. doi:10.1016/S0891-5520(03)00095-3. PMID 15081510.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Oldstone MBA (2009). Viruses, Plagues, and History: Past, Present and Future. Oxford University Press, USA. p. 315. ISBN 0-19-532731-4.
- ^ Goodpasture EW, Woodruff AM, Buddingh GJ (1931). "The cultivation of vaccine and other viruses in the chorioallantoic membrane of chick embryos". Science 74, pp. 371–372 PMID 17810781
- ^ Rosen FS (2004). "Isolation of poliovirus—John Enders and the Nobel Prize". New England Journal of Medicine, 351, pp. 1481–83 PMID 15470207
- ^ Magrath I (2009). "Lessons from clinical trials in African Burkitt lymphoma". Current Opinion in Oncology. 21 (5): 462–8. doi:10.1097/CCO.0b013e32832f3dcd. PMID 19620863.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Epstein, M. Anthony (2005). "1. The origins of EBV research: discovery and characterization of the virus". In Robertson, Earl S. (ed.). Epstein-Barr Virus. Trowbridge: Cromwell Press. pp. 1–14. ISBN 1904455034. Retrieved September 18, 2010.
- ^ Bornkamm GW (2009). "Epstein-Barr virus and the pathogenesis of Burkitt's lymphoma: more questions than answers". International Journal of Cancer. Journal International Du Cancer. 124 (8): 1745–55. doi:10.1002/ijc.24223. PMID 19165855.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Thorley-Lawson DA (2005). "EBV the prototypical human tumor virus—just how bad is it?". The Journal of Allergy and Clinical Immunology. 116 (2): 251–61, quiz 262. doi:10.1016/j.jaci.2005.05.038. PMID 16083776.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Oldstone MBA (2009). Viruses, Plagues, and History: Past, Present and Future. Oxford University Press, USA. p. 4. ISBN 0-19-532731-4.
- ^ Belongia EA, Naleway AL (2003). "Smallpox vaccine: the good, the bad, and the ugly". Clinical Medical Research. 1 (2): 87–92. doi:10.3121/cmr.1.2.87. PMC 1069029. PMID 15931293.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Shchelkunov SN (2009). "How long ago did smallpox virus emerge?". Archives of Virology. 154 (12): 1865–71. doi:10.1007/s00705-009-0536-0. PMID 19882103.
- ^ Mahy BWJ and Van Regenmortel MHV (2009). Desk Encyclopedia of Human and Medical Virology. Boston: Academic Press. p. 237. ISBN 0-12-375147-0.
- ^ Lane JM (2006). "Mass vaccination and surveillance/containment in the eradication of smallpox". Current Topics in Microbiology and Immunology. 304: 17–29. doi:10.1007/3-540-36583-4_2. PMID 16989262.
- ^ Tucker, Jonathan B. (2002). Scourge: the once and future threat of smallpox. New York: Grove Press. pp. 126–131. ISBN 0-8021-3939-6.
- ^ Oldstone MBA (2009). Viruses, Plagues, and History: Past, Present and Future. Oxford University Press, USA. p. 84. ISBN 0-19-532731-4.
- ^ Barré-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vézinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L (1983). "Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS)". Science. 220 (4599): 868–71. PMID 6189183.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Esparza J, Osmanov S (2003). "HIV vaccines: a global perspective". Current Molecular Medicine. 3 (3): 183–93. doi:10.2174/1566524033479825. PMID 12699356.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Mahy BWJ and Van Regenmoretel MHV (2009). Desk Encyclopedia of Human and Medical Virology. Boston: Academic Press. pp. 336–337. ISBN 0-12-375147-0.
- ^ Benjamin Weeks (2009). AIDS: The Biological Basis. Sudbury, Mass: Jones & Bartlett Publishers. pp. 15–21. ISBN 0-7637-6324-1.
- ^ Van Heuverswyn F, Peeters M (2007). "The origins of HIV and implications for the global epidemic". Current Infectious Disease Reports. 9 (4): 338–46. doi:10.1007/s11908-007-0052-x. PMID 17618555.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Paiardini M, Pandrea I, Apetrei C, Silvestri G (2009). "Lessons learned from the natural hosts of HIV-related viruses". Annual Review of Medicine. 60: 485–95. doi:10.1146/annurev.med.60.041807.123753. PMID 19630581.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Benjamin Weeks (2009). AIDS: The Biological Basis. Sudbury, Mass: Jones & Bartlett Publishers. p. 19. ISBN 0-7637-6324-1.
- ^ Sharp PM, Hahn BH (2010). "The evolution of HIV-1 and the origin of AIDS". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 365 (1552): 2487–94. doi:10.1098/rstb.2010.0031. PMC 2935100. PMID 20643738.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Frederick A Murphy (2008) "Discoverers and Discoveries", International Committee on Taxonomy of Viruses
- ^ Olafson P, MacCallum AD, Fox FH (1946). "An apparently new transmissible disease of cattle". The Cornell Veterinarian. 36: 205–13. PMID 20995890.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Peterhans E, Bachofen C, Stalder H, Schweizer M (2010). "Cytopathic bovine viral diarrhea viruses (BVDV): emerging pestiviruses doomed to extinction". Veterinary Research. 41 (6): 44. doi:10.1051/vetres/2010016. PMC 2850149. PMID 20197026.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bryans JT, Crowe ME, Doll ER, McCollum WH (1957). "Isolation of a filterable agent causing arteritis of horses and abortion by mares; its differentiation from the equine abortion (influenza) virus". The Cornell Veterinarian. 47 (1): 3–41. PMID 13397177.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Weller TH (1995). "Varicella-zoster virus: History, perspectives, and evolving concerns". Neurology. 45 (12 Suppl 8): S9–10. PMID 8545033.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c Schmidt AC, Johnson TR, Openshaw PJ, Braciale TJ, Falsey AR, Anderson LJ, Wertz GW, Groothuis JR, Prince GA, Melero JA, Graham BS (2004). "Respiratory syncytial virus and other pneumoviruses: a review of the international symposium—RSV 2003". Virus Research. 106 (1): 1–13. doi:10.1016/j.virusres.2004.06.008. PMID 15522442.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Griffin DE, Pan CH (2009). "Measles: old vaccines, new vaccines". Current Topics in Microbiology and Immunology. 330: 191–212. doi:10.1007/978-3-540-70617-5_10. PMID 19203111.
- ^ a b Tyrrell DA (1987). "The common cold—my favourite infection. The eighteenth Majority Stephenson memorial lecture". The Journal of General Virology. 68 ( Pt 8): 2053–61. PMID 3039038.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Zetterström R (2008). "Nobel Prize to Baruch Blumberg for the discovery of the aetiology of hepatitis B". Acta Paediatrica (Oslo, Norway : 1992). 97 (3): 384–7. doi:10.1111/j.1651-2227.2008.00669.x. PMID 18298788.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Yoshida M (1997). "Howard Temin memorial lectureship. Molecular biology of HTLV-1: deregulation of host cell gene expression and cell cycle". Leukemia : Official Journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 11 (1): 14–5. PMID 9001412.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Temin HM, Baltimore D (1972). "RNA-directed DNA synthesis and RNA tumor viruses". Advances in Virus Research. 17: 129–86. doi:10.1016/S0065-3527(08)60749-6. PMID 4348509.
{{cite journal}}:|access-date=requires|url=(help) - ^ Broder S (2010). "The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic". Antiviral Research. 85 (1): 1–18. doi:10.1016/j.antiviral.2009.10.002. PMC 2815149. PMID 20018391.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Peiris JS, Poon LL (2011). "Detection of SARS Coronavirus". Methods in Molecular Biology (Clifton, N.J.). 665: 369–82. doi:10.1007/978-1-60761-817-1_20. PMID 21116811.
- ^ Field H, Young P, Yob JM, Mills J, Hall L, Mackenzie J (2001). "The natural history of Hendra and Nipah viruses". Microbes and Infection / Institut Pasteur. 3 (4): 307–14. PMID 11334748.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Mahy BWJ (2009). Desk Encyclopedia of Human and Medical Virology. Boston: Academic Press. pp. 583–587. ISBN 0-12-375147-0.
- ^ Quoted in:Eskild Peterson; Ryan, Kenneth J.; Nafees Ahmad (2010). Sherris Medical Microbiology, Fifth Edition. McGraw-Hill Medical. p. 101. ISBN 0-07-160402-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Thurber RV (2009). "Current insights into phage biodiversity and biogeography". Curr. Opin. Microbiol. 12 (5): 582–7. doi:10.1016/j.mib.2009.08.008. PMID 19811946.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Breitbart M, Rohwer F (2005). "Here a virus, there a virus, everywhere the same virus?". Trends in Microbiology. 13 (6): 278–84. doi:10.1016/j.tim.2005.04.003. PMID 15936660.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c Suttle CA (2005). "Viruses in the sea". Nature. 437 (7057): 356–61. doi:10.1038/nature04160. PMID 16163346.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Sullivan MB, Coleman ML, Weigele P, Rohwer F, Chisholm SW (2005). "Three Prochlorococcus cyanophage genomes: signature features and ecological interpretations". Plos Biology. 3 (5): e144. doi:10.1371/journal.pbio.0030144. PMC 1079782. PMID 15828858.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Kurth R, Bannert N (2010). "Beneficial and detrimental effects of human endogenous retroviruses". Int. J. Cancer. 126 (2): 306–14. doi:10.1002/ijc.24902. PMID 19795446.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Blikstad V, Benachenhou F, Sperber GO, Blomberg J (2008). "Evolution of human endogenous retroviral sequences: a conceptual account". Cellular and Molecular Life Sciences : CMLS. 65 (21): 3348–65. doi:10.1007/s00018-008-8495-2. PMID 18818874.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kurth R, Bannert N (2010). "Beneficial and detrimental effects of human endogenous retroviruses". International Journal of Cancer. Journal International Du Cancer. 126 (2): 306–14. doi:10.1002/ijc.24902. PMID 19795446.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Leppard, Keith; Nigel Dimmock; Easton, Andrew (2007). Introduction to Modern Virology. Blackwell Publishing Limited. pp. 426–430. ISBN 1-4051-3645-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Skern T (2010). "100 years poliovirus: from discovery to eradication. A meeting report". Archives of Virology. 155 (9): 1371–81. doi:10.1007/s00705-010-0778-x. PMID 20683737.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Becsei-Kilborn E (2010). "Scientific discovery and scientific reputation: the reception of Peyton Rous' discovery of the chicken sarcoma virus". Journal of the History of Biology. 43 (1): 111–57. doi:10.1007/s10739-008-9171-y. PMID 20503720.
- ^ Gardner CL, Ryman KD (2010). "Yellow fever: a reemerging threat". Clinics in Laboratory Medicine. 30 (1): 237–60. doi:10.1016/j.cll.2010.01.001. PMID 20513550.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Zacks MA, Paessler S (2010). "Encephalitic alphaviruses". Veterinary Microbiology. 140 (3–4): 281–6. doi:10.1016/j.vetmic.2009.08.023. PMC 2814892. PMID 19775836.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Johnson CD, Goodpasture EW (1934). "An investigation of the etiology of mumps". The Journal of Experimental Medicine. 59 (1): 1–19. doi:10.1084/jem.59.1.1. PMC 2132344. PMID 19870227.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Misra UK, Kalita J (2010). "Overview: Japanese encephalitis". Progress in Neurobiology. 91 (2): 108–20. doi:10.1016/j.pneurobio.2010.01.008. PMID 20132860.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Melnick JL (1993). "The discovery of the enteroviruses and the classification of poliovirus among them". Biologicals : Journal of the International Association of Biological Standardization. 21 (4): 305–9. doi:10.1006/biol.1993.1088. PMID 8024744.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Martin, Malcolm A.; Knipe, David M.; Fields, Bernard N.; Howley, Peter M.; Griffin, Diane; Lamb, Robert (2007). Fields' virology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 2395. ISBN 0-7817-6060-7.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Douglass N, Dumbell K (1992). "Independent evolution of monkeypox and variola viruses". Journal of Virology. 66 (12): 7565–7. PMC 240470. PMID 1331540.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Cooper LZ (1985). "The history and medical consequences of rubella". Reviews of Infectious Diseases. 7 Suppl 1: S2–10. PMID 3890105.
- ^ Yap SF (2004). "Hepatitis B: review of development from the discovery of the "Australia Antigen" to end of the twentieth Century". The Malaysian Journal of Pathology. 26 (1): 1–12. PMID 16190102.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Epstein MA, Achong BG, Barr YM, Zajac B, Henle G, Henle W (1966). "Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji)". Journal of the National Cancer Institute. 37 (4): 547–59. PMID 4288580.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Karpas A (2004). "Human retroviruses in leukaemia and AIDS: reflections on their discovery, biology and epidemiology". Biological Reviews of the Cambridge Philosophical Society. 79 (4): 911–33. doi:10.1017/S1464793104006505. PMID 15682876.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Curtis N (2006). "Viral haemorrhagic fevers caused by Lassa, Ebola and Marburg viruses". Advances in Experimental Medicine and Biology. 582: 35–44. doi:10.1007/0-387-33026-7_4. PMID 16802617.
- ^ Hartman AL, Towner JS, Nichol ST (2010). "Ebola and marburg hemorrhagic fever". Clinics in Laboratory Medicine. 30 (1): 161–77. doi:10.1016/j.cll.2009.12.001. PMID 20513546.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kapikian AZ (2000). "The discovery of the 27-nm Norwalk virus: an historic perspective". The Journal of Infectious Diseases. 181 Suppl 2: S295–302. doi:10.1086/315584. PMID 10804141.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Bishop RF, Cameron DJ, Barnes GL, Holmes IH, Ruck BJ (1976). "The aetiology of diarrhoea in newborn infants". Ciba Foundation Symposium (42): 223–36. PMID 186236.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gust ID, Coulepis AG, Feinstone SM, Locarnini SA, Moritsugu Y, Najera R, Siegl G (1983). "Taxonomic classification of hepatitis A virus". Intervirology. 20 (1): 1–7. doi:10.1159/000149367. PMID 6307916.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cossart Y (1981). "Parvovirus B19 finds a disease". Lancet. 2 (8253): 988–9. PMID 6117755.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Feldmann H, Geisbert TW (2010). "Ebola haemorrhagic fever". Lancet. doi:10.1016/S0140-6736(10)60667-8. PMID 21084112.
{{cite journal}}: Unknown parameter|month=ignored (help)Epub ahead of print - ^ a b Gallo RC (2005). "History of the discoveries of the first human retroviruses: HTLV-1 and HTLV-2". Oncogene. 24 (39): 5926–30. doi:10.1038/sj.onc.1208980. PMID 16155599.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Montagnier L (2010). "25 years after HIV discovery: prospects for cure and vaccine". Virology. 397 (2): 248–54. doi:10.1016/j.virol.2009.10.045. PMID 20152474.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ De Bolle L, Naesens L, De Clercq E (2005). "Update on human herpesvirus 6 biology, clinical features, and therapy". Clinical Microbiology Reviews. 18 (1): 217–45. doi:10.1128/CMR.18.1.217-245.2005. PMC 544175. PMID 15653828.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M (1989). "Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome". Science. 244 (4902): 359–62. PMID 2523562.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Bihl F, Negro F (2010). "Hepatitis E virus: a zoonosis adapting to humans". The Journal of Antimicrobial Chemotherapy. 65 (5): 817–21. doi:10.1093/jac/dkq085. PMID 20335188.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Wild TF (2009). "Henipaviruses: a new family of emerging Paramyxoviruses". Pathologie-biologie. 57 (2): 188–96. doi:10.1016/j.patbio.2008.04.006. PMID 18511217.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Okamoto H (2009). "History of discoveries and pathogenicity of TT viruses". Current Topics in Microbiology and Immunology. 331: 1–20. doi:10.1007/978-3-540-70972-5_1. PMID 19230554.


