Electron transfer
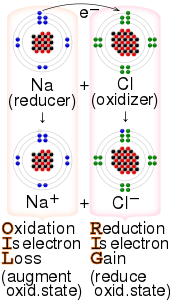
Electron transfer (ET) occurs when an electron relocates from an atom or molecule to another such chemical entity. ET is a mechanistic description of certain kinds of redox reactions involving transfer of electrons.
Electrochemical processes are ET reaction. ET reactions are relevant to photosynthesis and respiration. ET reactions commonly involve transition metal complexes,[2][3] In organic chemistry ET is a step in some commercial polymerization reactions. It is foundational to photoredox catalysis.
Classes of electron transfer
Inner-sphere electron transfer
In inner-sphere ET, the two redox centers are covalently linked during the ET. This bridge can be permanent, in which case the electron transfer event is termed intramolecular electron transfer. More commonly, however, the covalent linkage is transitory, forming just prior to the ET and then disconnecting following the ET event. In such cases, the electron transfer is termed intermolecular electron transfer. A famous example of an inner sphere ET process that proceeds via a transitory bridged intermediate is the reduction of [CoCl(NH3)5]2+ by [Cr(H2O)6]2+. In this case, the chloride ligand is the bridging ligand that covalently connects the redox partners.
Outer-sphere electron transfer
In outer-sphere ET reactions, the participating redox centers are not linked via any bridge during the ET event. Instead, the electron "hops" through space from the reducing center to the acceptor. Outer sphere electron transfer can occur between different chemical species or between identical chemical species that differ only in their oxidation state. The latter process is termed self-exchange. As an example, self-exchange describes the degenerate reaction between permanganate and its one-electron reduced relative manganate:
- [MnO4]− + [Mn*O4]2− → [MnO4]2− + [Mn*O4]−
In general, if electron transfer is faster than ligand substitution, the reaction will follow the outer-sphere electron transfer.
Often occurs when one/both reactants are inert or if there is no suitable bridging ligand.
A key concept of Marcus theory is that the rates of such self-exchange reactions are mathematically related to the rates of "cross reactions". Cross reactions entail partners that differ by more than their oxidation states. One example (of many thousands) is the reduction of permanganate by iodide to form iodine and, again, manganate.
Five steps of an outer sphere reaction
- 1. reactants diffuse together out of their solvent shells => precursor complex (requires work =wr)
- 2. changing bond lengths, reorganize solvent => activated complex
- 3. Electron transfer
- 4. Relaxation of bond lengths, solvent molecules => successor complex
- 5. Diffusion of products (requires work=wp)
Heterogeneous electron transfer
In heterogeneous electron transfer, an electron moves between a chemical species and a solid-state electrode. Theories addressing heterogeneous electron transfer have applications in electrochemistry and the design of solar cells.
Theory
The first generally accepted theory of ET was developed by Rudolph A. Marcus to address outer-sphere electron transfer and was based on a transition-state theory approach. The Marcus theory of electron transfer was then extended to include inner-sphere electron transfer by Noel Hush and Marcus. The resultant theory called Marcus-Hush theory, has guided most discussions of electron transfer ever since. Both theories are, however, semiclassical in nature, although they have been extended to fully quantum mechanical treatments by Joshua Jortner, Alexander M. Kuznetsov, and others proceeding from Fermi's golden rule and following earlier work in non-radiative transitions. Furthermore, theories have been put forward to take into account the effects of vibronic coupling on electron transfer; in particular, the PKS theory of electron transfer.[4]
Before 1991, ET in metalloproteins was thought to affect primarily the diffuse, averaged properties of the non-metal atoms forming an insulated barrier between the metals, but Beratan, Betts and Onuchic [5] subsequently showed that the ET rates are governed by the bond structures of the proteins -- that the electrons, in effect, tunnel through the bonds comprising the chain structure of the proteins. In fact, this discussion considers only the average of the dominant electron transport path(s) observed under experimental conditions, and while through bond couplings are clearly dominant for some artificial systems created to study electron transfer, many natural systems show clear evidence of highly multiple or 'average' coupling through the protein environment as if the identity or bonding of the atoms is less important than their density. It is important to note that electron tunnelling is not accurately conceived with singular defined paths -- that quantum tunnelling of an electron is the end result of the electron sampling all possible electron transfer 'paths' via its wavefunction, and that experiments report only on weighted averages of these paths.
See also
References
- ^ "Metals". Bitesize. BBC. Archived from the original on 2022-11-03.
- ^ Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ^ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- ^ Susan B. Piepho, Elmars R. Krausz, P. N. Schatz; J. Am. Chem. Soc., 1978, 100 (10), pp 2996–3005; Vibronic coupling model for calculation of mixed-valence absorption profiles; doi:10.1021/ja00478a011; Publication Date: May 1978
- ^ Beratan DN, Betts JN, Onuchic JN, Science 31 May 1991: Vol. 252 no. 5010 pp. 1285-1288; Protein electron transfer rates set by the bridging secondary and tertiary structure; doi:10.1126/science.1656523; Publication Date: May 1991
