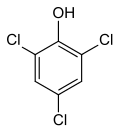
2,4,6-Trichlorophenol
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2,4,6-Trichlorophenol
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.633 | ||
| EC Number |
| ||
| RTECS number |
| ||
| UN number | 2020 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| C6H2Cl3OH/C6H3Cl3O | |||
| Molar mass | 197.45 g/mol | ||
| Appearance | yellow-whitish lumps or powder | ||
| Density | 1.675 g/cm3 | ||
| Melting point | 69 °C (156 °F; 342 K) | ||
| Boiling point | 246 °C (475 °F; 519 K) at 28 torr | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
2,4,6-Trichlorophenol, also known as TCP, phenaclor, Dowicide 2S, Dowcide 2S, omal, is a chlorinated phenol that has been used as a fungicide, herbicide, insecticide, antiseptic,[1] defoliant, and glue preservative.[2] It is a clear to yellowish crystalline solid with a strong, phenolic odor. It decomposes on heating to produce toxic and corrosive fumes including hydrogen chloride and chlorine.
Health effects
In animal models, consumption of 2,4,6-trichlorophenol leads to an increased incidence of lymphomas, leukemia, and liver cancer.[3][4] It is classified as Group B2 (probable human carcinogen) by the United States Environmental Protection Agency.[4] The technical grade of this substance may contain polychlorinated dibenzodioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), and other contaminants.[5]
Environmental effects
2,4,6-Trichlorophenol is an environmental pollutant that has been found in fresh water lakes such as the Great Lakes.[6]
See also
- Trichlorophenol (for other isomers).
References
- ^ Ogunniyi TAB, Oni PO, Juba A, Asaolu SO, and Kolawole DO (2000-01-05). "Disinfectants/antiseptics in the management of guinea worm ulcers in the rural areas". Acta Tropica. 74 (1): 33–38(6). doi:10.1016/S0001-706X(99)00057-1.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Safety data for 2,4,6-trichlorophenol". University of Oxford. 2005-09-05. Archived from the original on 14 October 2007. Retrieved 2007-11-16.
{{cite web}}: Italic or bold markup not allowed in:|publisher=(help); Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "2,4,6-Trichlorophenol". The Carcinogenic Potency Database Project, University of Berkeley. 2007-10-03. Archived from the original on 4 December 2007. Retrieved 2007-11-16.
{{cite web}}: Italic or bold markup not allowed in:|publisher=(help); Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b "2,4,6 Trichlorophenol". United States Environmental Protection Agency. Jan 2000. Retrieved 2007-11-16.
{{cite web}}: Italic or bold markup not allowed in:|publisher=(help) - ^ "2,4,6-Trichlorophenol". ICSC 1122. IPCS. Nov 1998. Retrieved 2007-11-16.
{{cite journal}}: Cite journal requires|journal=(help) - ^ TP Halappa Gowdal, John D Lock, and Ruth G Kurtz (Feb 1985). "A comprehensive study of risk assessment for a hazardous compound of public health concern". Water, Air, & Soil Pollution. 24 (2). doi:10.1007/BF00285444. Retrieved 2007-11-16.
{{cite journal}}: CS1 maint: multiple names: authors list (link)