Manganese lactate
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C 6H 10MnO 6 | |
| Molar mass | 233.08 g/mol |
| Appearance | Pink crystals |
| Very soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
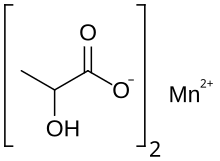
Manganese lactate is an organic chemical compound, a salt of manganese and lactic acid with the formula Mn(C3H5O3)2. The compound forms light pink crystals, soluble in water, forming crystalline hydrates.[1][2]
Synthesis
Dissolution of manganese carbonate in lactic acid solution:
- MnCO3 + 2C3H6O3 → Mn(C3H5O3)2 + CO2 + H2O
Physical properties
Manganese lactate forms light pink crystals.
Manganese lactate is soluble in water and ethanol.
Manganese lactate forms crystalline hydrates of composition Mn(C3H5O3)2•n H2O, where n = 2 and 3.[3] Each lactate is a bidentate ligand. Two water molecules coordinate directly to the metal as well, cis to each other, to complete the octahedral cluster. In the trihydrate, the third water molecule is involved in hydrogen-bonding further away in the crystal structure.[4]
Its dihydrate forms an orthorhombic crystal, the space group is P212121.[5]
The trihydrate is a monoclinic crystal, and the space group is P21.[6][7]
References
- ^ "Manganese(II) Lactate Trihydrate". American Elements. Retrieved 17 January 2022.
- ^ "Jost Chemical - Manganese Lactate, CAS Number 51877-53-3 (anh.)". Jost Chemical. Retrieved 17 January 2022.
- ^ "Manganese(II) lactate trihydrate". Sigma Aldrich. Retrieved 17 January 2022.
- ^ Ke, Zeng-Bo; Fan, Xin-Hui; Di, You-Ying; Chen, Feng-Ying; Zhang, Li-Jun; Yang, Ke; Li, Bing (March 2022). "Crystal Structures and Solution Chemical Properties of Two Lactate Complexes Mn[(C3H5O3)2(H2O)2]⋅H2O(s) and Cu(C3H5O3)2(s)". Journal of Molecular Structure. 1252: 132145. Bibcode:2022JMoSt125232145K. doi:10.1016/j.molstruc.2021.132145. S2CID 245214598.
- ^ Lis, T. (15 March 1982). "Structure of manganese(II) L-lactate dihydrate". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry. 38 (3): 937–939. doi:10.1107/S056774088200449X.
- ^ Singh, K. D.; Jain, S. C.; Sakore, T. D.; Biswas, A. B. (October 1975). "Crystal structure of some metal complexes of lactic acid". Zeitschrift für Kristallographie. 141 (5–6): 473–475. Bibcode:1975ZK....141..473S. doi:10.1524/zkri.1975.141.5-6.473.
- ^ Kemmitt, Tim; Mills, Ann M.; Gainsford, Graeme J. (2001). "The Formation of Manganese Carboxylates from MnO and MnO2 and their Application in Lithium Manganate Precursors: X-Ray Crystal Structure of Manganese Lactate Trihydrate". Australian Journal of Chemistry. 54 (1): 37–42. doi:10.1071/CH01012. Retrieved 17 January 2022.
