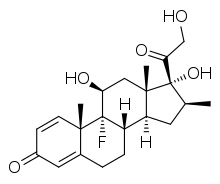
Betamethasone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Celestone, Eleuphrat, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682799 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, topical, intramuscular (IM) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Liver CYP3A4 |
| Elimination half-life | 36-54 hours |
| Excretion | Kidney (in urine) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.206 |
| Chemical and physical data | |
| Formula | C22H29FO5 |
| Molar mass | 392.461 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Betamethasone is a steroid medication.[3] It is used for a number of diseases including rheumatic disorders such as rheumatoid arthritis and systemic lupus erythematosus, skin diseases such as dermatitis and psoriasis, allergic conditions such as asthma and angioedema, preterm labor to speed the development of the baby's lungs, Crohn's disease, cancers such as leukemia, and along with fludrocortisone for adrenocortical insufficiency, among others.[3] It can be taken by mouth, injected into a muscle, or applied to the skin, typically in cream, lotion, or liquid forms.[3][4]
Serious side effects include an increased risk of infection, muscle weakness, severe allergic reactions, and psychosis.[3] Long-term use may cause adrenal insufficiency.[3] Stopping the medication suddenly following long-term use may be dangerous.[3] The cream commonly results in increased hair growth and skin irritation.[4] Betamethasone belongs to the glucocorticoid class of medication.[3] It is a stereoisomer of dexamethasone, the two compounds differing only in the spatial configuration of the methyl group at position 16 (see steroid nomenclature).[5]
Betamethasone was patented in 1958, and approved for medical use in the United States in 1961.[3][6] The cream and ointment are on the World Health Organization's List of Essential Medicines.[7] It is available as a generic medication.[3] In 2020, it was the 233rd most commonly prescribed medication in the United States, with more than 1 million prescriptions.[8][9]
Medical uses
Betamethasone is a corticosteroid that is available as a pill, by injection, and as an ointment, cream, lotion, gel, or aerosol (spray) for the skin, and a foam for the scalp.[10] When given by injection, anti-inflammatory effects begin in around two hours and last for seven days.[3]
It is used as a topical cream to relieve skin irritation, such as itching and flaking from eczema. It is used as a treatment for local psoriasis, as betamethasone dipropionate and salicylic acid, or as the combination calcipotriol/betamethasone dipropionate. Betamethasone sodium phosphate is used orally and via injection with the same indications as other steroids. Many betamethasone-based pharmaceuticals include the steroid as the valerate ester.
In a randomized controlled trial betamethasone was shown to reduce some of the ataxia (poor coordination) symptoms associated with ataxia telangiectasia (A-T) by 28-31%.[11]
Betamethasone is also used to stimulate fetal lung maturation in order to prevent infant respiratory distress syndrome (IRDS) and to decrease the incidence and mortality from intracranial hemorrhage in premature infants.
A cream with 0.05% betamethasone appears effective in treating phimosis in boys,[12] and often averts the need for circumcision.[13][14][15] It has replaced circumcision as the preferred treatment method for some physicians in the British National Health Service.[16][17]
Side effects
- Euphoria[18]
- Depression[18]
- Adrenal suppression[18]
- Hypertension[18]
- Groupings of fine blood vessels becoming prominent under the skin, petechiae[18]
- Excessive hair growth (hypertrichosis)[18]
- Ecchymoses[18]
Prolonged use of this medicine on extensive areas of skin, broken or raw skin, skin folds, or underneath airtight dressings may on rare occasions result in enough corticosteroid being absorbed to have side effects on other parts of the body; for example, by causing a decrease in the production of natural hormones by the adrenal glands.
Betamethasone is also used prior to delivery of a preterm baby to help prepare the lungs for breathing. However, because betamethasone crosses the placenta, which is required for its beneficial effects, it may also be associated with complications, such as hypoglycemia and leukocytosis in newborns exposed in utero.
When injected into the epidural space or the spine, it may cause serious side effects like loss of vision, stroke, and paralysis.[19]
Forms
Betamethasone is available in a number of compound forms: betamethasone dipropionate (branded as Diprosone, Diprolene, Celestamine, Procort (in Pakistan), and others), betamethasone sodium phosphate (branded as Bentelan in Italy) and betamethasone valerate (branded as Audavate, Betnovate, Celestone, Fucibet, and others). In the United States and Canada, betamethasone is mixed with clotrimazole and sold as Lotrisone and Lotriderm. It is also available in combination with salicylic acid (branded as Diprosalic) for using in psoriatic skin conditions. In some countries, it is also sold mixed with both clotrimazole and gentamicin to add an antibacterial agent to the mix.
Betamethasone sodium phosphate mixed with betamethasone acetate is available in the United States as Celestone Soluspan.[20]
See also
References
- ^ a b "Betamethasone Use During Pregnancy". Drugs.com. 30 December 2019. Retrieved 29 March 2020.
- ^ "Betamethasone 500 microgram Soluble Tablets - Summary of Product Characteristics (SmPC)". (emc). 5 April 2018. Retrieved 29 March 2020.
- ^ a b c d e f g h i j "Betamethasone". The American Society of Health-System Pharmacists. Archived from the original on 8 December 2015. Retrieved 2 December 2015.
- ^ a b "Betamethasone topical". The American Society of Health-System Pharmacists. Archived from the original on 8 December 2015. Retrieved 2 December 2015.
- ^ Antignac JP, Le Bizec B, Monteau F, Andre F (January 2002). "Differentiation of betamethasone and dexamethasone using liquid chromatography/positive electrospray tandem mass spectrometry and multivariate statistical analysis". Journal of Mass Spectrometry. 37 (1): 69–75. Bibcode:2002JMSp...37...69A. doi:10.1002/jms.260. PMID 11813313.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 485. ISBN 9783527607495.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- ^ "Betamethasone - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- ^ "Betamethasone Topical". MedlinePlus. 19 March 2020. Retrieved 29 March 2020.
- ^ Zannolli R, Buoni S, Betti G, Salvucci S, Plebani A, Soresina A, et al. (September 2012). "A randomized trial of oral betamethasone to reduce ataxia symptoms in ataxia telangiectasia". Movement Disorders. 27 (10): 1312–1316. doi:10.1002/mds.25126. PMID 22927201. S2CID 23696748.
- ^ Moreno G, Corbalán J, Peñaloza B, Pantoja T (September 2014). "Topical corticosteroids for treating phimosis in boys". The Cochrane Database of Systematic Reviews. 9 (9): CD008973. doi:10.1002/14651858.CD008973.pub2. PMID 25180668.
- ^ Van Howe RS (October 1998). "Cost-effective treatment of phimosis". Pediatrics. 102 (4): E43. doi:10.1542/peds.102.4.e43. PMID 9755280. Archived from the original on 19 August 2009. A review of estimated costs and complications of 3 phimosis treatments.
- ^ Esposito C, Centonze A, Alicchio F, Savanelli A, Settimi A (April 2008). "Topical steroid application versus circumcision in pediatric patients with phimosis: a prospective randomized placebo controlled clinical trial". World Journal of Urology. 26 (2). Springer Science and Business Media LLC: 187–190. doi:10.1007/s00345-007-0231-2. PMID 18157674. S2CID 8922151.
- ^ Zampieri N, Corroppolo M, Zuin V, Bianchi S, Camoglio FS (April 2007). "Phimosis and topical steroids: new clinical findings". Pediatric Surgery International. 23 (4): 331–335. doi:10.1007/s00383-007-1878-x. PMID 17308904. S2CID 22849471.
- ^ Berdeu D, Sauze L, Ha-Vinh P, Blum-Boisgard C (February 2001). "Cost-effectiveness analysis of treatments for phimosis: a comparison of surgical and medicinal approaches and their economic effect". BJU International. 87 (3): 239–244. doi:10.1046/j.1464-410x.2001.02033.x. PMID 11167650. S2CID 2161551.
- ^ Chu CC, Chen KC, Diau GY (September 1999). "Topical steroid treatment of phimosis in boys". The Journal of Urology. 162 (3 Pt 1): 861–863. doi:10.1097/00005392-199909010-00078. PMID 10458396.
- ^ a b c d e f g "betamethasone" (PDF). F.A. Davis. 2017. Archived (PDF) from the original on 8 September 2017. Retrieved 7 March 2017.
- ^ "FDA Drug Safety Communication: FDA requires label changes to warn of rare but serious neurologic problems after epidural corticosteroid injections for pain". FDA. 23 April 2014. Archived from the original on 11 August 2016. Retrieved 15 August 2016.
- ^ "Celestone Soluspan- betamethasone acetate and betamethasone sodium phosphate injection, suspension". DailyMed. 18 November 2019. Retrieved 29 March 2020.
External links
- "Betamethasone". Drug Information Portal. U.S. National Library of Medicine.
- "Betamethasone sodium phosphate". Drug Information Portal. U.S. National Library of Medicine.
- "Betamethasone acetate mixture with betamethasone sodium phosphate". Drug Information Portal. U.S. National Library of Medicine.
