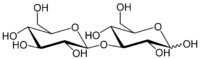
Laminaribiose
Appearance
| |
| Names | |
|---|---|
| IUPAC name
3-β-D-Glucopyranosyl-(1→3)-D-glucose
| |
| Systematic IUPAC name
(2R,3S,4R,5R)-2,4,5,6-Tetrahydroxy-3-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}hexanal | |
| Other names
3-β-D-Glucosyl-D-glucose
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C12H22O11 | |
| Molar mass | 342.30 g/mol |
| Density | 1.768 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Laminaribiose C12H22O11 is a disaccharide which is used notably in the agricultural field and as an antiseptic. It is in general obtained by hydrolysis or by acetolysis of natural polysaccharides of plant origin.[1] It is also a product of the caramelization of glucose. [2]
References
[edit]- ^ US 6632940
- ^ Sugisawa, Hirqshi; Edo, Hiroshi (1966). "The Thermal Degradation of Sugars I. Thermal Polymerization of Glucose". Journal of Food Science. 31 (4): 561. doi:10.1111/j.1365-2621.1966.tb01905.x.
