LRP2
Low density lipoprotein receptor-related protein 2 also known as LRP-2 or megalin is a protein which in humans is encoded by the LRP2 gene.[5][6][7]
Function
LRP2 was identified as the antigen of rat experimental membranous nephropathy (Heyman nephritis) and originally named gp330 and subsequently megalin[8] and later LRP2. LRP2/megalin is a multiligand binding receptor found in the plasma membrane of many absorptive epithelial cells. LRP2 is an approximately 600kDa (4665 amino acids) transmembrane glycoprotein with structural similarities to the low density lipoprotein receptor (LDLR).[9] LRP2 has a NPXY motif that is the binding site for Dab2 to initiate clathrin-mediated endocytosis.[10] LRP2 forms a homodimer that changes conformation in response to pH.[11] At pH 7.5 (extracellular pH), LRP2 is considered active, with the leucine loops in an open conformation to allow ligands to bind.[11] At acidic endosomal pHs, the leucine loops collapse to prevent ligands binding.[11]
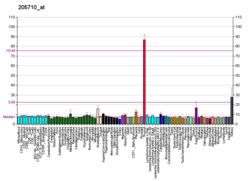
LRP2 is expressed in epithelial cells of the thyroid (thyrocytes), where it can serve as a receptor for the protein thyroglobulin (Tg).[12] LRP2 is also expressed on the apical surface of epithelial cells in the proximal tubule of the kidney.[9] It is highly expressed in the first segment (S1) of the proximal tubule, with decreasing expression in the second (S2) and third segment (S3) of the proximal tubule.[9] LRP2 is also expressed in podocytes, and antigenic response to LRP2 in podocytes is the primary cause of Heymann nephritis in rats.[8]
LRP2/megalin functions to mediate endocytosis of ligands leading to degradation in lysosomes or transcytosis. LRP2/megalin can also form complexes with CUBAM, the cubilin and amnionless complex. Those complexes are able to reabsorb several molecules and can be inhibited by sodium maleate. LRP2 and CUBAM are responsible for the uptake of most of the filtered proteins that escape the glomerular filtration barrier in the proximal tubule of the kidney.[13][14] The endocytic capacity of the proximal tubule cells is dictated by the combined function of LRP2, CUBAM, and Dab2.[14]
The epithelial cells of the proximal tubule are highly polarized and have a robust apical endocytic pathway, subapical compartmentalization, and large endocytic capacity.[13] This pathway is mediated by LRP2 and CUBAM, where Dab2 binds to the cytoplasmic tails of both LRP2 and CUBAM to initiate clathrin-coated endocytosis.[9][13] Once internalized, the endosomes release their clathrin coats and fuse with a dense subapical network of tubules to recycle receptors back to the apical surface.[9] As the endosomes acidify, LRP2 release its cargo and undergoes a conformational change which collapses the binding pockets to inhibit ligands rebinding to LRP2 in the endosomes.[11] Recycling of the LRP2 occurs from apical vacuoles with Rab11a positive endosomes, also referred to as dense apical tubules.[15] The vesicles are directed back to the plasma membrane where LRP2 undergoes another conformational change due to the change in pH and becomes active again.[11][15] According to LRP2/megalin kinetic modeling, the rate of megalin recycling and return to the apical surface from dense apical tubules has the largest impact on determining the overall endocytic capacity of proximal tubule cells and the endocytic rate of LRP2.[15] The fraction of LRP2 at the apical surface is important for the continued ability of the protein to reabsorb filtered proteins in the proximal tubule to maintain the robust endocytic capacity of these cells.[9][13][14]
Clinical significance
Disfunction in the LRP2-mediated endocytic trafficking and endocytic capacity in the proximal tubule can result in low molecular weight proteinuria, which is a hallmark of many diseases.[13]
Mutations in the LRP2 gene are associated with Donnai-Barrow syndrome.[16]
Dent's Disease (Dent 1) is associated with a drop in LRP2/megalin protein level in the proximal tubule with no detectable decrease in mRNA, suggesting that the loss of ClC-5, the gene mutated in Dent's Disease, shortens the half-life of the LRP2 receptor.[17][18] The loss of ClC-5 has been found to delay the early endosome maturation in the LRP2 trafficking in the proximal tubule cells.[18]
LRP2 has been shown to play a role in the development of nephrotoxic acute kidney injury (AKI) by mediating the uptake of nephrotoxic agents.[19] However, there have been no further studies to show the functional importance of LRP2 or CUBAM in the progression of AKI.
A decrease in LRP2 receptor expression has been reported in animal models of acute and chronic kidney diseases.[19]
Interactions
LRP2 has been shown to associate with the following proteins in the plasma membrane/cytosol of cells:
- CUBAM,[9][13]
- DAB2,[20]
- DLG4,[21][22]
- GIPC1,[21][23][24]
- ITGB1BP1,[21]
- LDL-receptor-related protein associated protein,[23][25]
- LDLRAP1,[26]
- MAGI1,[27]
- MAPK8IP1,[21][24]
- MAPK8IP2,[21][24]
- NOS1AP,[21] and
- SYNJ2BP.[21]
LRP2 has been shown to bind to the following ligands:
- Albumin[9]
- Vitamin D-binding protein[9]
- Hemoglobin[9]
- Myoglobin[9]
- Angiotensin II[9]
- Insulin[9]
- Leptin[9]
- Prolactin[9]
- Epidermal Growth Factor[9]
- Cathepsin B[9]
- Immunoglobulin light chains[9]
- Ca2+[9]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000081479 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000027070 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Entrez Gene: LRP2 low density lipoprotein-related protein 2".
- ^ Korenberg JR, Argraves KM, Chen XN, Tran H, Strickland DK, Argraves WS (July 1994). "Chromosomal localization of human genes for the LDL receptor family member glycoprotein 330 (LRP2) and its associated protein RAP (LRPAP1)". Genomics. 22 (1): 88–93. doi:10.1006/geno.1994.1348. PMID 7959795.
- ^ Farquhar MG (September 1995). "The unfolding story of megalin (gp330): now recognized as a drug receptor". The Journal of Clinical Investigation. 96 (3): 1184. doi:10.1172/JCI118149. PMC 185736. PMID 7657789.
- ^ a b Farquhar MG, Saito A, Kerjaschki D, Orlando RA (July 1995). "The Heymann nephritis antigenic complex: megalin (gp330) and RAP". Journal of the American Society of Nephrology. 6 (1): 35–47. doi:10.1681/ASN.V6135. PMID 7579068.
- ^ a b c d e f g h i j k l m n o p q r s Eshbach ML, Weisz OA (February 2017). "Receptor-Mediated Endocytosis in the Proximal Tubule". Annual Review of Physiology. 79 (1): 425–448. doi:10.1146/annurev-physiol-022516-034234. PMC 5512543. PMID 27813828.
- ^ Gallagher H, Oleinikov AV, Fenske C, Newman DJ (March 2004). "The adaptor disabled-2 binds to the third psi xNPxY sequence on the cytoplasmic tail of megalin". Biochimie. 86 (3): 179–182. doi:10.1016/j.biochi.2004.03.001. PMID 15134832.
- ^ a b c d e Beenken A, Cerutti G, Brasch J, Guo Y, Sheng Z, Erdjument-Bromage H, et al. (February 2023). "Structures of LRP2 reveal a molecular machine for endocytosis". Cell. 186 (4): 821–836.e13. doi:10.1016/j.cell.2023.01.016. PMC 9993842. PMID 36750096.
- ^ Zheng G, Marino' M, Zhao J, McCluskey RT (March 1998). "Megalin (gp330): a putative endocytic receptor for thyroglobulin (Tg)". Endocrinology. 139 (3): 1462–1465. doi:10.1210/endo.139.3.5978. PMID 9492085.
- ^ a b c d e f Weisz OA (July 2021). "Endocytic adaptation to functional demand by the kidney proximal tubule". The Journal of Physiology. 599 (14): 3437–3446. doi:10.1113/JP281599. PMC 8715547. PMID 34036593.
- ^ a b c Long KR, Rbaibi Y, Bondi CD, Ford BR, Poholek AC, Boyd-Shiwarski CR, et al. (January 2022). "Cubilin-, megalin-, and Dab2-dependent transcription revealed by CRISPR/Cas9 knockout in kidney proximal tubule cells". American Journal of Physiology. Renal Physiology. 322 (1): F14–F26. doi:10.1152/ajprenal.00259.2021. PMC 8698540. PMID 34747197.
- ^ a b c Shipman KE, Long KR, Cowan IA, Rbaibi Y, Baty CJ, Weisz OA (2022-10-28). "An Adaptable Physiological Model of Endocytic Megalin Trafficking in Opossum Kidney Cells and Mouse Kidney Proximal Tubule". Function. 3 (6): zqac046. doi:10.1093/function/zqac046. PMC 9614980. PMID 36325513.
- ^ Kantarci S, Al-Gazali L, Hill RS, Donnai D, Black GC, Bieth E, et al. (August 2007). "Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes". Nature Genetics. 39 (8): 957–959. doi:10.1038/ng2063. PMC 2891728. PMID 17632512.
- ^ Shipman KE, Weisz OA (2020-09-14). "Making a Dent in Dent Disease". Function. 1 (2): zqaa017. doi:10.1093/function/zqaa017. PMC 7519470. PMID 33015630.
- ^ a b Shipman KE, Baty CJ, Long KR, Rbaibi Y, Cowan IA, Gerges M, et al. (April 2023). "Impaired Endosome Maturation Mediates Tubular Proteinuria in Dent Disease Cell Culture and Mouse Models". Journal of the American Society of Nephrology. 34 (4): 619–640. doi:10.1681/ASN.0000000000000084. PMID 36758125. S2CID 256737627.
- ^ a b Nielsen R, Christensen EI, Birn H (January 2016). "Megalin and cubilin in proximal tubule protein reabsorption: from experimental models to human disease". Kidney International. 89 (1): 58–67. doi:10.1016/j.kint.2015.11.007. PMID 26759048.
- ^ Oleinikov AV, Zhao J, Makker SP (May 2000). "Cytosolic adaptor protein Dab2 is an intracellular ligand of endocytic receptor gp600/megalin". The Biochemical Journal. 347 (Pt 3): 613–621. doi:10.1042/0264-6021:3470613. PMC 1220996. PMID 10769163.
- ^ a b c d e f g Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, et al. (August 2000). "Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction". The Journal of Biological Chemistry. 275 (33): 25616–25624. doi:10.1074/jbc.M000955200. PMID 10827173.
- ^ Larsson M, Hjälm G, Sakwe AM, Engström A, Höglund AS, Larsson E, et al. (July 2003). "Selective interaction of megalin with postsynaptic density-95 (PSD-95)-like membrane-associated guanylate kinase (MAGUK) proteins". The Biochemical Journal. 373 (Pt 2): 381–391. doi:10.1042/BJ20021958. PMC 1223512. PMID 12713445.
- ^ a b Lou X, McQuistan T, Orlando RA, Farquhar MG (April 2002). "GAIP, GIPC and Galphai3 are concentrated in endocytic compartments of proximal tubule cells: putative role in regulating megalin's function". Journal of the American Society of Nephrology. 13 (4): 918–927. doi:10.1681/ASN.V134918. PMID 11912251.
- ^ a b c Petersen HH, Hilpert J, Militz D, Zandler V, Jacobsen C, Roebroek AJ, Willnow TE (February 2003). "Functional interaction of megalin with the megalinbinding protein (MegBP), a novel tetratrico peptide repeat-containing adaptor molecule". Journal of Cell Science. 116 (Pt 3): 453–461. doi:10.1242/jcs.00243. PMID 12508107.
- ^ Orlando RA, Farquhar MG (April 1994). "Functional domains of the receptor-associated protein (RAP)". Proceedings of the National Academy of Sciences of the United States of America. 91 (8): 3161–3165. Bibcode:1994PNAS...91.3161O. doi:10.1073/pnas.91.8.3161. PMC 43535. PMID 7512726.
- ^ Nagai M, Meerloo T, Takeda T, Farquhar MG (December 2003). "The adaptor protein ARH escorts megalin to and through endosomes". Molecular Biology of the Cell. 14 (12): 4984–4996. doi:10.1091/mbc.E03-06-0385. PMC 284800. PMID 14528014.
- ^ Patrie KM, Drescher AJ, Goyal M, Wiggins RC, Margolis B (April 2001). "The membrane-associated guanylate kinase protein MAGI-1 binds megalin and is present in glomerular podocytes". Journal of the American Society of Nephrology. 12 (4): 667–677. doi:10.1681/ASN.V124667. PMID 11274227.
Further reading
- Longoni M, Kantarci S, Donnai D, Pober BR (August 2008). "Donnai-Barrow Syndrome". In Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, Gripp KW, et al. (eds.). GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle. PMID 20301732.
- Farquhar MG, Saito A, Kerjaschki D, Orlando RA (July 1995). "The Heymann nephritis antigenic complex: megalin (gp330) and RAP". Journal of the American Society of Nephrology. 6 (1): 35–47. doi:10.1681/ASN.V6135. PMID 7579068.
- Farquhar MG (September 1995). "The unfolding story of megalin (gp330): now recognized as a drug receptor". The Journal of Clinical Investigation. 96 (3): 1184. doi:10.1172/JCI118149. PMC 185736. PMID 7657789.
- Christensen EI, Birn H (April 2002). "Megalin and cubilin: multifunctional endocytic receptors". Nature Reviews. Molecular Cell Biology. 3 (4): 256–266. doi:10.1038/nrm778. PMID 11994745. S2CID 21893726.
- Saito A, Takeda T, Hama H, Oyama Y, Hosaka K, Tanuma A, et al. (October 2005). "Role of megalin, a proximal tubular endocytic receptor, in the pathogenesis of diabetic and metabolic syndrome-related nephropathies: protein metabolic overload hypothesis". Nephrology. 10 (Suppl): S26–S31. doi:10.1111/j.1440-1797.2005.00453.x. PMID 16174284. S2CID 42737684.
- Fisher CE, Howie SE (August 2006). "The role of megalin (LRP-2/Gp330) during development". Developmental Biology. 296 (2): 279–297. doi:10.1016/j.ydbio.2006.06.007. PMID 16828734.
- Christensen EI, Gliemann J, Moestrup SK (October 1992). "Renal tubule gp330 is a calcium binding receptor for endocytic uptake of protein". The Journal of Histochemistry and Cytochemistry. 40 (10): 1481–1490. doi:10.1177/40.10.1382088. PMID 1382088.
- Raychowdhury R, Niles JL, McCluskey RT, Smith JA (June 1989). "Autoimmune target in Heymann nephritis is a glycoprotein with homology to the LDL receptor". Science. 244 (4909): 1163–1165. Bibcode:1989Sci...244.1163R. doi:10.1126/science.2786251. PMID 2786251.
- Orlando RA, Farquhar MG (April 1994). "Functional domains of the receptor-associated protein (RAP)". Proceedings of the National Academy of Sciences of the United States of America. 91 (8): 3161–3165. Bibcode:1994PNAS...91.3161O. doi:10.1073/pnas.91.8.3161. PMC 43535. PMID 7512726.
- Kounnas MZ, Chappell DA, Strickland DK, Argraves WS (July 1993). "Glycoprotein 330, a member of the low density lipoprotein receptor family, binds lipoprotein lipase in vitro". The Journal of Biological Chemistry. 268 (19): 14176–14181. doi:10.1016/S0021-9258(19)85224-9. PMID 7686151.
- Kounnas MZ, Loukinova EB, Stefansson S, Harmony JA, Brewer BH, Strickland DK, Argraves WS (June 1995). "Identification of glycoprotein 330 as an endocytic receptor for apolipoprotein J/clusterin". The Journal of Biological Chemistry. 270 (22): 13070–13075. doi:10.1074/jbc.270.22.13070. PMID 7768901.
- Korenberg JR, Argraves KM, Chen XN, Tran H, Strickland DK, Argraves WS (July 1994). "Chromosomal localization of human genes for the LDL receptor family member glycoprotein 330 (LRP2) and its associated protein RAP (LRPAP1)". Genomics. 22 (1): 88–93. doi:10.1006/geno.1994.1348. PMID 7959795.
- Lundgren S, Hjälm G, Hellman P, Ek B, Juhlin C, Rastad J, et al. (June 1994). "A protein involved in calcium sensing of the human parathyroid and placental cytotrophoblast cells belongs to the LDL-receptor protein superfamily". Experimental Cell Research. 212 (2): 344–350. doi:10.1006/excr.1994.1153. PMID 8187828.
- Moestrup SK, Nielsen S, Andreasen P, Jørgensen KE, Nykjaer A, Røigaard H, et al. (August 1993). "Epithelial glycoprotein-330 mediates endocytosis of plasminogen activator-plasminogen activator inhibitor type-1 complexes". The Journal of Biological Chemistry. 268 (22): 16564–16570. doi:10.1016/S0021-9258(19)85456-X. PMID 8344937.
- Hjälm G, Murray E, Crumley G, Harazim W, Lundgren S, Onyango I, et al. (July 1996). "Cloning and sequencing of human gp330, a Ca(2+)-binding receptor with potential intracellular signaling properties". European Journal of Biochemistry. 239 (1): 132–137. doi:10.1111/j.1432-1033.1996.0132u.x. PMID 8706697.
- Willnow TE, Hilpert J, Armstrong SA, Rohlmann A, Hammer RE, Burns DK, Herz J (August 1996). "Defective forebrain development in mice lacking gp330/megalin". Proceedings of the National Academy of Sciences of the United States of America. 93 (16): 8460–8464. Bibcode:1996PNAS...93.8460W. doi:10.1073/pnas.93.16.8460. PMC 38693. PMID 8710893.
- Cui S, Verroust PJ, Moestrup SK, Christensen EI (October 1996). "Megalin/gp330 mediates uptake of albumin in renal proximal tubule". The American Journal of Physiology. 271 (4 Pt 2): F900–F907. doi:10.1152/ajprenal.1996.271.4.F900. PMID 8898021.
- Lundgren S, Carling T, Hjälm G, Juhlin C, Rastad J, Pihlgren U, et al. (March 1997). "Tissue distribution of human gp330/megalin, a putative Ca(2+)-sensing protein". The Journal of Histochemistry and Cytochemistry. 45 (3): 383–392. doi:10.1177/002215549704500306. PMID 9071320.
- Birn H, Verroust PJ, Nexo E, Hager H, Jacobsen C, Christensen EI, Moestrup SK (October 1997). "Characterization of an epithelial approximately 460-kDa protein that facilitates endocytosis of intrinsic factor-vitamin B12 and binds receptor-associated protein". The Journal of Biological Chemistry. 272 (42): 26497–26504. doi:10.1074/jbc.272.42.26497. PMID 9334227.