Leishmania tropica
| Leishmania tropica | |
|---|---|

| |
| Promastigotes of Leishmania tropica. Giemsa stain, 10×100. | |
| Scientific classification | |
| Domain: | Eukaryota |
| Phylum: | Euglenozoa |
| Class: | Kinetoplastea |
| Order: | Trypanosomatida |
| Genus: | Leishmania |
| Species: | L. tropica
|
| Binomial name | |
| Leishmania tropica Wright, 1903
| |

| |
| Red = cutaneous L. tropica[1] | |
Leishmania tropica is a flagellate parasite and the cause of anthroponotic[dubious – discuss] cutaneous leishmaniasis in humans.[2] This parasite is restricted to Afro-Eurasia and is a common cause of infection in Afghanistan, Iran, Syria, Yemen, Algeria, Morocco, and northern India.[3]
History
The first description of Leishmania tropica was done in 1903 by James Homer Wright, an American pathologist. In 1914, it was suggested that L. tropica should be divided into two subspecies, namely L. tropica minor and L. tropica major, based on the size of the parasites found in skin lesions.[3] Later, these two subspecies turned out to be epidemiologically different and were correlated to different types of lesions. L. tropica minor causes dry nodular lesions in urban environments, while L. tropica major causes wet ulcerating lesions in rural regions.[4] Bray et al. therefore proposed in 1973 that the subspecies should be considered as two separate species, L. tropica major became L. major, and L. tropica minor became L. tropica,[4] which is the naming that is still being used.[3]
Biology
The parasite has an interesting biology since it is very heterogeneous biochemically, serologically, and genetically compared to other Leishmania species.[5] The different strains have been shown to cause different patterns of pathogenicity and humoral immune responses in BALB/c mice models.[3] Fusion and hybridization of different L. tropica strains can be efficiently induced in vitro by exposure of promastigotes (a stage of the life cycle) to DNA damage stress.[6]
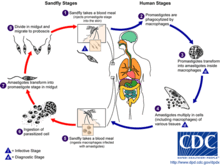
Life cycle
Leishmania species alternate between two main life forms: intracellular amastigotes in the sandfly – the vector – and extracellular motile promastigotes in the mammal – the host.[8] In the mammalian host, promastigotes are introduced into the skin by the bite of a sandfly. After being taken up by phagocytes, they transform into intracellular amastigotes and stay in this form during the remaining life cycle in the mammalian host.[8] Through simple division, they can multiply and proceed to infect other phagocytotic cells. Later, depending partly on the immunity of the host, the infection can become symptomatic and result in leishmaniasis.[7] Sand flies become infected by ingesting phagocytes with Leishmania from a mammalian host. Then, in the sandfly stage, the process differs between Leishmania species. In the life cycle of L. tropica, it develops back into the promastigote stage inside the midgut of the sandfly vector and migrates to the proboscis of the sandfly, whereafter the life cycle can repeat itself.[7]
Hosts and vectors
Humans are the main reservoir hosts of L. tropica.[9] Rock hyraxes (Procavia capensis)[9] are a possible reservoir in Israel.[10] Natural infection by L. tropica has also been demonstrated in domestic dogs,[10] red foxes, golden jackals, gundis, and other species of wild rodents.[3] The main sandfly[10] vector for L. tropica is Phlebotomus sergenti. Other reported vector species are P. arabicus, P. guggisbergi, P. chabaudi, P. rossi, and P. saevus.[3]
Clinical manifestations
L. tropica causes a broad spectrum of leishmaniasis forms in humans. Most common is a variant called dry-type cutaneous leishmaniasis. After an incubation period lasting more than 2 months, a small brownish nodular lesion will appear with a slowly extending plaque reaching a size of 1–2 centimetres (0.39–0.79 in) after 6 months. This will heal after about 1 year but leaves a scar.[11] Other forms of the disease, which occur more rarely in humans, include visceral leishmaniasis, post-kala-azar dermal leishmaniasis (a variant of visceral leishmaniasis), viscerotropic leishmaniasis, and leishmaniasis recidivans (a variant of cutaneous leishmaniasis).[3]
Dogs are known to rarely suffer from visceral, skin, and mucosal infection with this species.[10] In cats asymptomatic infection is thought to be common.[10] Skin and/or mucosal infection is the most common form, with or without visceral infection.[10] Feline visceral infection may occur alone.[10]
Leishmaniasis recidivans
This rare variant of cutaneous leishmaniasis is caused solely by L. tropica in the Old World and by L. braziliensis in the New World. It causes a slowly progressing lesion, usually on the face, and is characterized by the development of papules or nodules which form mostly around or in the site of primary healed lesions. Lesions of leishmaniasis recidivans stay many years and rarely respond to treatment, thus causing disfigurement and becoming destructive with the years.[12]
References
- ^ Aoun, K.; Bouratbine, A. (2014). "Cutaneous Leishmaniasis in North Africa: a review". Parasite. 21. EDP Sciences: 14. doi:10.1051/parasite/2014014. PMC 3952656. PMID 24626301.
- ^ Karimi, Taiebeh; Sharifi, Iraj; Aflatoonian, Mohammad Reza; Aflatoonian, Behnaz; Mohammadi, Mohammad Ali; Salarkia, Ehsan; Babaei, Zahra; Zarinkar, Farzaneh; Sharifi, Fatemeh; Hatami, Nima; Khosravi, Ahmad; Eskandari, Arsalan; Solimani, Elyas; Shafiee, Mehdi; Mozaffari, Masoumeh (2021). "A long-lasting emerging epidemic of anthroponotic cutaneous leishmaniasis in southeastern Iran: population movement and peri-urban settlements as a major risk factor". Parasites & Vectors. 14 (1): 122. doi:10.1186/s13071-021-04619-3. ISSN 1756-3305. PMC 7903377. PMID 33627184. S2CID 232036410.
- ^ a b c d e f g Rostamian, Mosayeb; Niknam, Hamid M. (2019). "Leishmania tropica: What we know from its experimental models". Advances in Parasitology. 104: 6–7 – via Elsevier Science Direct.
- ^ a b Bray, R.S.; Ashford, R.W.; Bray, M.A. (1973). "The parasite causing cutaneous leishmaniasis in Ethiopia". Transactions of the Royal Society of Tropical Medicine and Hygiene. 67 (3): 345–348. doi:10.1016/0035-9203(73)90111-9. PMID 4778189 – via Elsevier Science Direct.
- ^ Magill, Alan J (2013-01-01). "99 - Leishmaniasis". In Magill, Alan J.; Hill, David R; Solomon, Tom; Ryan, Edward T (eds.). Hunter's Tropical Medicine and Emerging Infectious Disease (Ninth ed.). London: W.B. Saunders. pp. 739–760. doi:10.1016/b978-1-4160-4390-4.00099-0. ISBN 978-1-4160-4390-4. Retrieved 2022-12-15.
- ^ Louradour, I; Ferreira, TR; Duge, E; Karunaweera, N; Paun, A; Sacks, D (2022-01-07). "Stress conditions promote Leishmania hybridization in vitro marked by expression of the ancestral gamete fusogen HAP2 as revealed by single-cell RNA-seq". eLife. 11. doi:10.7554/eLife.73488. PMC 8794473. PMID 34994687. S2CID 245835348. e73488.
- ^ a b c CDC - Centers for Disease Control and Prevention (2020-02-18). "CDC - Leishmaniasis - Biology". CDC. Retrieved 2022-12-15.
- ^ a b Gossage, Sharon M; Rogers, Matthew E; Bates, Paul A (2003). "Two separate growth phases during the development of Leishmania in sand flies: implications for understanding the life cycle". International Journal for Parasitology. 33 (10): 1027–1034. doi:10.1016/S0020-7519(03)00142-5. PMC 2839921. PMID 13129524.
- ^ a b Ashford, Richard W. (1996). "Leishmaniasis reservoirs and their significance in control". Clinics in Dermatology. 14 (5): 523–532. doi:10.1016/0738-081X(96)00041-7. PMID 8889331.
- ^ a b c d e f g Iowa State University; Institute for Cooperation in Animal Biologics; Iowa State University College of Veterinary Medicine; World Organisation for Animal Health/OIE Collaborating Centre for Diagnosis of Animal Disease and Vaccine Evaluation in the Americas; OIE Collaborating Centre for Day-One Veterinary Competencies and Continuing Education; United States Department of Agriculture (2017). "Leishmaniasis" (PDF).
- ^ Meymandi, Simin; Dabiri, Shahriar; Dabiri, Darya; Crawford, Richard I.; Kharazmi, Arsalan (2004). "A quantitative study of epidermal Langerhans cells in cutaneous leishmaniasis caused by Leishmania tropica". International Journal of Dermatology. 43 (11): 819–823. doi:10.1111/j.1365-4632.2004.02359.x. ISSN 0011-9059. PMID 15533064. S2CID 22132211.
- ^ Sharifi, Iraj; Fekri, Ali Reza; Aflatoonian, Mohammad Reza; Khamesipour, Ali; Mahboudi, Fereidoun; Dowlati, Yahya; Nadim, Abolhassan; Modabber, Farrokh (2010). "Leishmaniasis recidivans among school children in Bam, South-east Iran, 1994-2006". International Journal of Dermatology. 49 (5): 557–561. doi:10.1111/j.1365-4632.2010.04419.x. ISSN 1365-4632. PMID 20534092. S2CID 8747858.
Further reading
- Saroufim, Maya; Charafeddine, Khalil; Issa, Grace (October 2014). "Ongoing Epidemic of Cutaneous Leishmaniasis among Syrian Refugees, Lebanon". Emerging Infectious Diseases. 20 (10): 1712–1715. doi:10.3201/eid2010.140288. PMC 4193275. PMID 25279543.
- Solomon, Michal; Schwartz, Eli; Pavlotsky, Felix (Aug 2014). "Leishmania tropica in children: A retrospective study". Journal of the American Academy of Dermatology. 71 (2): 271–7. doi:10.1016/j.jaad.2013.12.047. PMID 24775403.
- Krayter, Lena; Alam, Mohammad Zahangir; Rhajaoui, Mohamed; Schnur, Lionel F. (December 2014). "Multilocus Microsatellite Typing reveals intra-focal genetic diversity among strains of Leishmania tropica in Chichaoua Province, Morocco". Infection, Genetics and Evolution. 28: 233–239. doi:10.1016/j.meegid.2014.09.037. PMID 25308380.
- Hammoudeh, Nour; Kweider, Mahmoud; Abbady, Abdul-Qader; Soukkarieh, Chadi (Oct 2014). "Sequencing and Gene Expression Analysis of Leishmania tropica LACK Gene" (PDF). Iranian Journal of Parasitology. 9 (4): 574–583. PMC 4345098. PMID 25759740. Retrieved 9 February 2015.
- Mahmoudzadeh-Niknam H, Kiaei SS, Iravani D (2007). "Leishmania tropica infection, in comparison to Leishmania major, induces lower delayed type hypersensitivity in BALB/c mice". Korean Journal Parasitology. 45 (2): 103–9. doi:10.3347/kjp.2007.45.2.103. PMC 2526302. PMID 17570972.
External links
 Media related to Leishmania tropica at Wikimedia Commons
Media related to Leishmania tropica at Wikimedia Commons- Leishmaniasis – General Information US Center for Disease Control and Prevention
- Cancer Web
