Chemistry of ascorbic acid
| |

| |
| Names | |
|---|---|
| IUPAC name
(5R)-[(1S)-1,2-Dihydroxyethyl]-3,4-dihydroxyfuran-2(5H)-one
| |
| Other names
Vitamin C
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| E number | E300 (antioxidants, ...) |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H8O6 | |
| Molar mass | 176.124 g·mol−1 |
| Appearance | White or light yellow solid |
| Density | 1.65 g/cm3 |
| Melting point | 190 to 192 °C (374 to 378 °F; 463 to 465 K) decomposes |
| 330 g/L | |
| Solubility | Insoluble in diethyl ether, chloroform, benzene, petroleum ether, oils, fats |
| Solubility in ethanol | 20 g/L |
| Solubility in glycerol | 10 g/L |
| Solubility in propylene glycol | 50 g/L |
| Acidity (pKa) | 4.10 (first), 11.6 (second) |
| Pharmacology | |
| A11GA01 (WHO) G01AD03 (WHO), S01XA15 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
11.9 g/kg (oral, rat)[1] |
| Safety data sheet (SDS) | JT Baker |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ascorbic acid is an organic compound with formula C
6H
8O
6, originally called hexuronic acid. It is a white solid, but impure samples can appear yellowish. It dissolves well in water to give mildly acidic solutions. It is a mild reducing agent.
Ascorbic acid exists as two enantiomers (mirror-image isomers), commonly denoted "l" (for "levo") and "d" (for "dextro"). The l isomer is the one most often encountered: it occurs naturally in many foods, and is one form ("vitamer") of vitamin C, an essential nutrient for humans and many animals. Deficiency of vitamin C causes scurvy, formerly a major disease of sailors in long sea voyages. It is used as a food additive and a dietary supplement for its antioxidant properties. The "d" form can be made via chemical synthesis but has no significant biological role.
History
The antiscorbutic properties of certain foods were demonstrated in the 18th century by James Lind. In 1907, Axel Holst and Theodor Frølich discovered that the antiscorbutic factor was a water-soluble chemical substance, distinct from the one that prevented beriberi. Between 1928 and 1932, Albert Szent-Györgyi isolated a candidate for this substance, which he called it "hexuronic acid", first from plants and later from animal adrenal glands. In 1932 Charles Glen King confirmed that it was indeed the antiscorbutic factor.
In 1933, sugar chemist Walter Norman Haworth, working with samples of "hexuronic acid" that Szent-Györgyi had isolated from paprika and sent him in the previous year, deduced the correct structure and optical-isomeric nature of the compound, and in 1934 reported its first synthesis.[2][3] In reference to the compound's antiscorbutic properties, Haworth and Szent-Györgyi proposed to rename it "a-scorbic acid" for the compound, and later specifically l-ascorbic acid.[4] Because of their work, in 1937 two Nobel Prizes: in Chemistry and in Physiology or Medicine were awarded to Haworth and Szent-Györgyi, respectively.
Independently, ascorbic acid was synthetized in 1933 by Tadeusz Reichstein (the Nobel Prize laureate in Physiology or Medicine in 1950).[5]
Chemical properties
Acidity
Ascorbic acid is a furan-based lactone of 2-ketogluconic acid. It contains an adjacent enediol adjacent to the carbonyl. This −C(OH)=C(OH)−C(=O)− structural pattern is characteristic of reductones, and increases the acidity of one of the enol hydroxyl groups. The deprotonated conjugate base is the ascorbate anion, which is stabilized by electron delocalization that results from resonance between two forms:
For this reason, ascorbic acid is much more acidic than would be expected if the compound contained only isolated hydroxyl groups.
Salts
The ascorbate anion forms salts, such as sodium ascorbate, calcium ascorbate, and potassium ascorbate.
Esters
Ascorbic acid can also react with organic acids as an alcohol forming esters such as ascorbyl palmitate and ascorbyl stearate.
Nucleophilic attack
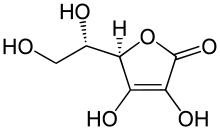
Nucleophilic attack of ascorbic acid on a proton results in a 1,3-diketone:
Oxidation
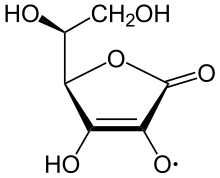

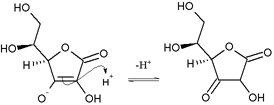
The ascorbate ion is the predominant species at typical biological pH values. It is a mild reducing agent and antioxidant. It is oxidized with loss of one electron to form a radical cation and then with loss of a second electron to form dehydroascorbic acid. It typically reacts with oxidants of the reactive oxygen species, such as the hydroxyl radical.
Ascorbic acid is special because it can transfer a single electron, owing to the resonance-stabilized nature of its own radical ion, called semidehydroascorbate. The net reaction is:
- RO• + C
6H
7O−
6 → RO− + C6H7O•
6 → ROH + C6H6O6[6]
On exposure to oxygen, ascorbic acid will undergo further oxidative decomposition to various products including diketogulonic acid, xylonic acid, threonic acid and oxalic acid.[7]
Reactive oxygen species are damaging to animals and plants at the molecular level due to their possible interaction with nucleic acids, proteins, and lipids. Sometimes these radicals initiate chain reactions. Ascorbate can terminate these chain radical reactions by electron transfer. The oxidized forms of ascorbate are relatively unreactive and do not cause cellular damage.
However, being a good electron donor, excess ascorbate in the presence of free metal ions can not only promote but also initiate free radical reactions, thus making it a potentially dangerous pro-oxidative compound in certain metabolic contexts.
Ascorbic acid and its sodium, potassium, and calcium salts are commonly used as antioxidant food additives. These compounds are water-soluble and, thus, cannot protect fats from oxidation: For this purpose, the fat-soluble esters of ascorbic acid with long-chain fatty acids (ascorbyl palmitate or ascorbyl stearate) can be used as food antioxidants.
Other reactions
It creates volatile compounds when mixed with glucose and amino acids in 90 °C.[8]
It is a cofactor in tyrosine oxidation.[9]
Uses
Food additive
The main use of l-ascorbic acid and its salts is as food additives, mostly to combat oxidation. It is approved for this purpose in the EU with E number E300,[10] USA,[11] Australia, and New Zealand.[12]
Dietary supplement
Another major use of l-ascorbic acid is as dietary supplement.
Niche, non-food uses
- Ascorbic acid is easily oxidized and so is used as a reductant in photographic developer solutions (among others) and as a preservative.
- In fluorescence microscopy and related fluorescence-based techniques, ascorbic acid can be used as an antioxidant to increase fluorescent signal and chemically retard dye photobleaching.[13]
- It is also commonly used to remove dissolved metal stains, such as iron, from fiberglass swimming pool surfaces.
- In plastic manufacturing, ascorbic acid can be used to assemble molecular chains more quickly and with less waste than traditional synthesis methods.[14]
- Heroin users are known to use ascorbic acid as a means to convert heroin base to a water-soluble salt so that it can be injected.[15]
- As justified by its reaction with iodine, it is used to negate the effects of iodine tablets in water purification. It reacts with the sterilized water, removing the taste, color, and smell of the iodine. This is why it is often sold as a second set of tablets in most sporting goods stores as Potable Aqua-Neutralizing Tablets, along with the potassium iodide tablets.
- Intravenous high-dose ascorbate is being used as a chemotherapeutic and biological response modifying agent.[16] Currently it is still under clinical trials.[17]
- It is sometimes used as a urinary acidifier to enhance the antiseptic effect of methenamine.[18][19]
Synthesis
Natural biosynthesis of vitamin C occurs in many plants, and animals, by a variety of processes.
Industrial preparation
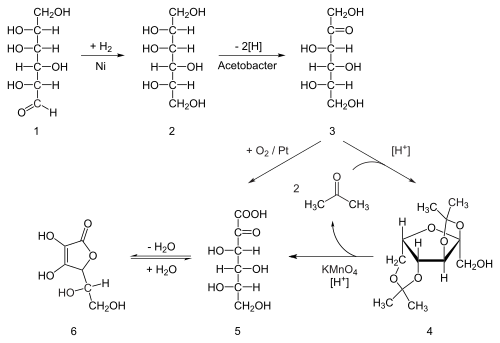
Eighty percent of the world's supply of ascorbic acid is produced in China.[20] Ascorbic acid is prepared in industry from glucose in a method based on the historical Reichstein process. In the first of a five-step process, glucose is catalytically hydrogenated to sorbitol, which is then oxidized by the microorganism Acetobacter suboxydans to sorbose. Only one of the six hydroxy groups is oxidized by this enzymatic reaction. From this point, two routes are available. Treatment of the product with acetone in the presence of an acid catalyst converts four of the remaining hydroxyl groups to acetals. The unprotected hydroxyl group is oxidized to the carboxylic acid by reaction with the catalytic oxidant TEMPO (regenerated by sodium hypochlorite – bleaching solution). Historically, industrial preparation via the Reichstein process used potassium permanganate as the bleaching solution. Acid-catalyzed hydrolysis of this product performs the dual function of removing the two acetal groups and ring-closing lactonization. This step yields ascorbic acid. Each of the five steps has a yield larger than 90%.[21]
A more biotechnological process, first developed in China in the 1960s, but further developed in the 1990s, bypasses the use of acetone-protecting groups. A second genetically modified microbe species, such as mutant Erwinia, among others, oxidises sorbose into 2-ketogluconic acid (2-KGA), which can then undergo ring-closing lactonization via dehydration. This method is used in the predominant process used by the ascorbic acid industry in China, which supplies 80% of world's ascorbic acid.[22] American and Chinese researchers are competing to engineer a mutant that can carry out a one-pot fermentation directly from glucose to 2-KGA, bypassing both the need for a second fermentation and the need to reduce glucose to sorbitol.[23]
There exists a d-ascorbic acid, which does not occur in nature but can be synthesized artificially. To be specific, l-ascorbate is known to participate in many specific enzyme reactions that require the correct enantiomer (l-ascorbate and not d-ascorbate).[24] l-Ascorbic acid has a specific rotation of [α]20
D = +23°.[25]
Determination
The traditional way to analyze the ascorbic acid content is the process of titration with an oxidizing agent, and several procedures have been developed.
The popular iodometry approach uses iodine in the presence of a starch indicator. Iodine is reduced by ascorbic acid, and, when all the ascorbic acid has reacted, the iodine is then in excess, forming a blue-black complex with the starch indicator. This indicates the end-point of the titration.
As an alternative, ascorbic acid can be treated with iodine in excess, followed by back titration with sodium thiosulfate using starch as an indicator.[26]
This iodometric method has been revised to exploit reaction of ascorbic acid with iodate and iodide in acid solution. Electrolyzing the solution of potassium iodide produces iodine, which reacts with ascorbic acid. The end of process is determined by potentiometric titration in a manner similar to Karl Fischer titration. The amount of ascorbic acid can be calculated by Faraday's law.
Another alternative uses N-bromosuccinimide (NBS) as the oxidizing agent, in the presence of potassium iodide and starch. The NBS first oxidizes the ascorbic acid; when the latter is exhausted, the NBS liberates the iodine from the potassium iodide, which then forms the blue-black complex with starch.
See also
- Colour retention agent
- Erythorbic acid: a diastereomer of ascorbic acid.
- Mineral ascorbates: salts of ascorbic acid
- Acids in wine
References
- ^ Safety (MSDS) data for ascorbic acid. University of Oxford
- ^ Story of Vitamin C's chemical discovery. Profiles.nlm.nih.gov. Retrieved on 2012-12-04.
- ^ Davies MB, Austin J, Partridge DA (1991). Vitamin C: Its Chemistry and Biochemistry. The Royal Society of Chemistry. p. 48. ISBN 0-85186-333-7.
- ^ Svirbelf JL, Szent-Györgyi A (April 25, 1932), "The Chemical Nature Of Vitamin C" (PDF), Science, 75 (1944): 357–8, Bibcode:1932Sci....75..357K, doi:10.1126/science.75.1944.357-a, PMID 17750032, S2CID 33277683. Part of the National Library of Medicine collection. Accessed January 2007
- ^ "Tadeus Reichstein. Biographical". Nobel Prize Outreach AB. Retrieved 19 April 2023.
- ^ Caspi R (Aug 19, 2009), "MetaCyc Compound: monodehydroascorbate radical", MetaCyc, retrieved 2014-12-08
- ^ Gaonkar AG, McPherson A (2016-04-19). Ingredient Interactions: Effects on Food Quality, Second Edition. ISBN 9781420028133.
- ^ Seck, S.; Crouzet, J. (1981). "Formation of Volatile Compounds in Sugar-Phenylalanine and Ascorbic Acid-Phenylalanine Model Systems during Heat Treatment". Journal of Food Science. 46 (3): 790–793. doi:10.1111/j.1365-2621.1981.tb15349.x.
- ^ Sealock RR, Goodland RL, Sumerwell WN, Brierly JM (May 1952). "The role of ascorbic acid in the oxidation of L-Tyrosine by guinea pig liver extracts" (PDF). The Journal of Biological Chemistry. 196 (2): 761–7. doi:10.1016/S0021-9258(19)52407-3. PMID 12981016.
- ^ UK Food Standards Agency: "Current EU approved additives and their E Numbers". Retrieved 2011-10-27.
- ^ US Food and Drug Administration: "Listing of Food Additives Status Part I". Food and Drug Administration. Archived from the original on 2012-01-17. Retrieved 2011-10-27.
- ^ Australia New Zealand Food Standards Code"Standard 1.2.4 – Labelling of ingredients". Retrieved 2011-10-27.
- ^ Widengren J, Chmyrov A, Eggeling C, Löfdahl PA, Seidel CA (January 2007). "Strategies to improve photostabilities in ultrasensitive fluorescence spectroscopy". The Journal of Physical Chemistry A. 111 (3): 429–40. Bibcode:2007JPCA..111..429W. doi:10.1021/jp0646325. PMID 17228891.
- ^ Vitamin C, water have benefits for plastic manufacturing, Reliable Plant Magazine, 2007, archived from the original on 2007-09-27, retrieved 2007-06-25
- ^ Beynon CM, McVeigh J, Chandler M, Wareing M, Bellis MA (December 2007). "The impact of citrate introduction at UK syringe exchange programmes: a retrospective cohort study in Cheshire and Merseyside, UK". Harm Reduction Journal. 4 (1): 21. doi:10.1186/1477-7517-4-21. PMC 2245922. PMID 18072971.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "The Riordan IVC Protocol for Adjunctive Cancer Care: Intravenous Ascorbate as a Chemotherapeutic and Biological Response Modifying Agent" (PDF). Riordan Clinic Research Institut. February 2013. Archived (PDF) from the original on 2022-10-09. Retrieved 2 February 2014.
{{cite journal}}: Cite journal requires|journal=(help) - ^ "High-Dose Vitamin C (PDQ®): Human/Clinical Studies". National Cancer Institute. 2013-02-08. Retrieved 2 February 2014.
- ^ Strom, J. Grady; Jun, H. Won (1993). "Effect of urine pH and ascorbic acid on the rate of conversion of methenamine to formaldehyde". Biopharmaceutics & Drug Disposition. 14 (1): 61–69. doi:10.1002/bdd.2510140106. PMID 8427945. S2CID 11151179.
- ^ Nahata, M. C.; Cummins, B. A.; McLeod, D. C.; Schondelmeyer, S. W.; Butler, R. (1982). "Effect of urinary acidifiers on formaldehyde concentration and efficacy with methenamine therapy". European Journal of Clinical Pharmacology. 22 (3): 281–284. doi:10.1007/bf00545228. PMID 7106162. S2CID 31796137.
- ^ Weiss R (May 20, 2007), "Tainted Chinese Imports Common", Washington Post, retrieved 2010-04-25
- ^ Eggersdorfer, M.; et al. "Vitamins". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_443. ISBN 978-3527306732.
- ^ China's grip on key food additive. The Christian Science Monitor. 2007-07-20. Retrieved on 2012-12-04.
- ^ BASF’s description of vitamin C—developments in production methods. Archived January 30, 2012, at the Wayback Machine. competition-commission.org.uk.
- ^ Rosa, Júlio César Câmara; Colombo, Lívia Tavares; Alvim, Mariana Caroline Tocantins; Avonce, Nelson; Van Dijck, Patrick; Passos, Flávia Maria Lopes (2013-06-22). "Metabolic engineering of Kluyveromyces lactis for L-ascorbic acid (vitamin C) biosynthesis". Microbial Cell Factories. 12: 59. doi:10.1186/1475-2859-12-59. ISSN 1475-2859. PMC 3699391. PMID 23799937.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Davies MB, Austin, John A., Partridge, David A. (1991). Vitamin C : its chemistry and biochemistry. Cambridge [Cambridgeshire]: Royal Society of Chemistry. ISBN 9780851863337.
- ^ "A Simple Test for Vitamin C" (PDF). School Science Review. 83 (305): 131. 2002. Archived from the original (PDF) on July 4, 2016.
Further reading
- Clayden J, Greeves N, Warren S, Wothers P (2001). Organic Chemistry. Oxford University Press. ISBN 0-19-850346-6.
- Davies MB, Austin J, Partridge DA (1991). Vitamin C: Its Chemistry and Biochemistry. Royal Society of Chemistry. ISBN 0-85186-333-7.
- Coultate TP (1996). Food: The Chemistry of Its Components (3rd ed.). Royal Society of Chemistry. ISBN 0-85404-513-9.
- Gruenwald J, Brendler T, Jaenicke C, eds. (2004). PDR for Herbal Medicines (3rd ed.). Montvale, New Jersey: Thomson PDR. ISBN 9781563635120.
- McMurry J (2008). Organic Chemistry (7e ed.). Thomson Learning. ISBN 978-0-495-11628-8.
External links
- International Chemical Safety Card 0379
- SIDS Initial Assessment Report for L-Ascorbic acid from the Organisation for Economic Co-operation and Development (OECD)
- IPCS Poisons Information Monograph (PIM) 046
- Interactive 3D-structure of vitamin C with details on the x-ray structure