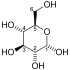
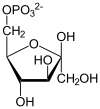
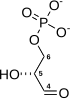
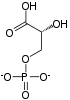
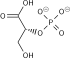
Fructose 1,6-bisphosphate

| |
| Names | |
|---|---|
| IUPAC name
1,6-Di-O-phosphono-β-D-fructofuranose
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.985 |
| KEGG | |
| MeSH | fructose-1,6-diphosphate |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H14O12P2 | |
| Molar mass | 340.116 |
| Pharmacology | |
| C01EB07 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Fructose 1,6-bisphosphate, also known as Harden-Young ester, is fructose sugar phosphorylated on carbons 1 and 6 (i.e., is a fructosephosphate). The β-D-form of this compound is common in cells.[1] Upon entering the cell, most glucose and fructose is converted to fructose 1,6-bisphosphate.[2][3]
In glycolysis
Fructose 1,6-bisphosphate lies within the glycolysis metabolic pathway and is produced by phosphorylation of fructose 6-phosphate. It is, in turn, broken down into two compounds: glyceraldehyde 3-phosphate and dihydroxyacetone phosphate. It is an allosteric activator of pyruvate kinase through distinct interactions of binding and allostery at the enzyme's catalytic site [4]
| β-D-fructose 6-phosphate | 6-phosphofructo-1-kinase | β-D-fructose 1,6-bisphosphate | Fructose-bisphosphate aldolase | D-glyceraldehyde 3-phosphate | dihydroxyacetone phosphate | |||

|
|

|
+ | 
| ||||
| ATP | ADP | |||||||

|

| |||||||
| Pi | H2O | |||||||
| Fructose 1,6-bisphosphatase | Fructose-bisphosphate aldolase | |||||||
Compound C05345 at KEGG Pathway Database. Enzyme 2.7.1.11 at KEGG Pathway Database. Enzyme 3.1.3.11 at KEGG Pathway Database. Compound C05378 at KEGG Pathway Database. Enzyme 4.1.2.13 at KEGG Pathway Database. Compound C00111 at KEGG Pathway Database. Compound C00118 at KEGG Pathway Database.
The numbering of the carbon atoms indicates the fate of the carbons according to their position in fructose 6-phosphate.
Click on genes, proteins and metabolites below to link to respective articles.[§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "GlycolysisGluconeogenesis_WP534".
Isomerism
Fructose 1,6-bisphosphate has only one biologically active isomer, the β-D-form. There are many other isomers, analogous to those of fructose.
Iron chelation
Fructose 1,6-bis(phosphate) has also been implicated in the ability to bind and sequester Fe(II), a soluble form of iron whose oxidation to the insoluble Fe(III) is capable of generating reactive oxygen species via Fenton chemistry. The ability of fructose 1,6-bis(phosphate) to bind Fe(II) may prevent such electron transfers, and thus act as an antioxidant within the body. Certain neurodegenerative diseases, like Alzheimer's and Parkinson's, have been linked to metal deposits with high iron content, although it is uncertain whether Fenton chemistry plays a substantial role in these diseases, or whether fructose 1,6-bis(phosphate) is capable of mitigating those effects.[5]
See also
References
- ^ Alfarouk, Khalid O.; Verduzco, Daniel; Rauch, Cyril; Muddathir, Abdel Khalig; Bashir, Adil H. H.; Elhassan, Gamal O.; Ibrahim, Muntaser E.; Orozco, Julian David Polo; Cardone, Rosa Angela; Reshkin, Stephan J.; Harguindey, Salvador (18 December 2014). "Glycolysis, tumor metabolism, cancer growth and dissemination. A new pH-based etiopathogenic perspective and therapeutic approach to an old cancer question". Oncoscience. 1 (12): 777–802. doi:10.18632/oncoscience.109. PMC 4303887. PMID 25621294.
- ^ Berg, Jeremy M.; Tymoczko, Stryer (2002). Biochemistry (5th ed.). New York: W.H. Freeman and Company. ISBN 0-7167-3051-0.
- ^ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- ^ Ishwar, Arjun (2015). "Distinguishing the Interactions in the Fructose 1,6-Bisphosphate Binding Site of Human Liver Pyruvate Kinase That Contribute to Allostery". Biochemistry. 54 (7): 1516–1524. doi:10.1021/bi501426w. PMC 5286843. PMID 25629396.
- ^ Bajic, Aleksandar; Zakrzewska J; Godjevac D; Andjus P; Jones DR; Spasic M; Spasojevic I (2011). "Relevance of the ability of fructose 1,6-bis(phosphate) to sequester ferrous but not ferric ions". Carbohydrate Research. 346 (3): 416–420. doi:10.1016/j.carres.2010.12.008. PMID 21232735.
External links
 Media related to Fructose 1,6-bisphosphate at Wikimedia Commons
Media related to Fructose 1,6-bisphosphate at Wikimedia Commons