Barium carbide
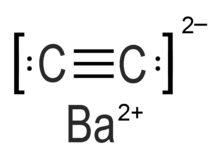
| |
| Names | |
|---|---|
| IUPAC name
Barium ethynediide
| |
| Other names
Barium acetylide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
| |
| |
| Properties | |
| BaC2 | |
| Molar mass | 161.35 g/mol |
| Appearance | black crystalline solid |
| Density | 3.75 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Barium carbide (also referred to as barium ethynediide or barium acetylide)[1] is a chemical compound in the carbide family having the chemical formula BaC2.[2]
Preparation
Barium carbide can be synthesized as an impure compound by reducing barium carbonate powder with metallic magnesium in the presence of carbon-14.[3] Carbon-14 containing barium carbide can also be made by reducing 14C carbon dioxide with hot barium metal at 600°C.[4] These methods are used because of their high yield, and because the carbide is used to make acetylene. (Carbon-14 is not something to turn into a waste product.) It can also be prepared by heating a barium amalgam and carbon powder mixture in a hydrogen current. The pure compound is prepared by reducing barium oxide with carbon at high temperature.[5]
Properties
Barium carbide reacts similarly to calcium carbide,[6] but it's more fusible. When exposed to extreme heat, the barium will evaporate leaving behind crystals of graphite. It can also absorb the carbon in a solution at high temperature.[5]
Hazards
Barium carbide can cause damage to the GI tract and irritation in the skin and eyes.[1]
References
- ^ a b "Barium acetylide | C2Ba | ChemSpider". chemspider.com. Retrieved 2019-12-17.
- ^ "Barium Carbide". American Elements. Retrieved 2019-12-11.
- ^ Mishin, V. I.; Georgievskij, S. S.; Eksel', L. M.; Koval', A. I.; Afanas'eva, L. A.; Puchkov, L. D.; Ulybin, V. B. (1989-12-07). "Method for preparation of barium carbide labelled by carbon 14" (in Russian).
- ^ Arrol, W. J.; Glascock, R. (1948). "308. The conversion of carbon dioxide into acetylene on the scale of 2—20 micromoles". J. Chem. Soc. 3: 1534–1537. doi:10.1039/JR9480001534. PMID 18101450.
- ^ a b "Barium Carbide, BaC2". barium.atomistry.com. Retrieved 2019-12-11.
- ^ "Carbide". InfoPlease. Retrieved 2019-12-11.
