Tin(II) oxide
| |

| |

| |
| Names | |
|---|---|
| IUPAC name
Tin(II) oxide
| |
| Other names
Stannous oxide
Tin monoxide | |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.040.439 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| SnO | |
| Molar mass | 134.709 g/mol |
| Appearance | black or red powder when anhydrous, white when hydrated |
| Density | 6.45 g/cm3 |
| Melting point | 1,080 °C (1,980 °F; 1,350 K)[1] |
| insoluble | |
| −19.0·10−6 cm3/mol | |
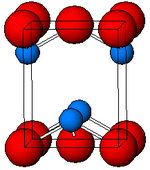
| Structure | |
| tetragonal | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
56 J·mol−1·K−1[2] |
Std enthalpy of
formation (ΔfH⦵298) |
−285 kJ·mol−1[2] |
| Hazards | |
| Flash point | Non-flammable |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
none[3] |
REL (Recommended)
|
TWA 2 mg/m3[3] |
IDLH (Immediate danger)
|
N.D.[3] |
| Safety data sheet (SDS) | ICSC 0956 |
| Related compounds | |
Other anions
|
Tin sulfide Tin selenide Tin telluride |
Other cations
|
Carbon monoxide Silicon monoxide Germanium(II) oxide Lead(II) oxide |
| Tin dioxide | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tin(II) oxide (stannous oxide) is a compound with the formula SnO. It is composed of tin and oxygen where tin has the oxidation state of +2. There are two forms, a stable blue-black form and a metastable red form.
Preparation and reactions

Blue-black SnO can be produced by heating the tin(II) oxide hydrate, SnO·xH2O (x<1) precipitated when a tin(II) salt is reacted with an alkali hydroxide such as NaOH.[4]
Metastable, red SnO can be prepared by gentle heating of the precipitate produced by the action of aqueous ammonia on a tin(II) salt.[4]
SnO may be prepared as a pure substance in the laboratory, by controlled heating of tin(II) oxalate (stannous oxalate) in the absence of air or under a CO2 atmosphere. This method is also applied to the production of ferrous oxide and manganous oxide.[5][6]
- SnC2O4·2H2O → SnO + CO2 + CO + 2 H2O
Tin(II) oxide burns in air with a dim green flame to form SnO2.[4]
- 2 SnO + O2 → 2 SnO2
When heated in an inert atmosphere initially disproportionation occurs giving Sn metal and Sn3O4 which further reacts to give SnO2 and Sn metal.[4]
- 4SnO → Sn3O4 + Sn
- Sn3O4 → 2SnO2 + Sn
SnO is amphoteric, dissolving in strong acid to give tin(II) salts and in strong base to give stannites containing Sn(OH)3−.[4] It can be dissolved in strong acid solutions to give the ionic complexes Sn(OH2)32+ and Sn(OH)(OH2)2+, and in less acid solutions to give Sn3(OH)42+.[4] Note that anhydrous stannites, e.g. K2Sn2O3, K2SnO2 are also known.[7][8][9] SnO is a reducing agent and is thought to reduce copper(I) to metallic clusters in the manufacture of so-called "copper ruby glass".[10]
Structure
Black, α-SnO adopts the tetragonal PbO layer structure containing four coordinate square pyramidal tin atoms.[11] This form is found in nature as the rare mineral romarchite.[12] The asymmetry is usually simply ascribed to a sterically active lone pair; however, electron density calculations show that the asymmetry is caused by an antibonding interaction of the Sn(5s) and the O(2p) orbitals.[13] The electronic structure and chemistry of the lone pair determines most of the properties of the material.[14]
Non-stoichiometry has been observed in SnO.[15]
The electronic band gap has been measured between 2.5eV and 3eV.[16]
Uses
The dominant use of stannous oxide is as a precursor in manufacturing of other, typically divalent, tin compounds or salts. Stannous oxide may also be employed as a reducing agent and in the creation of ruby glass.[17] It has a minor use as an esterification catalyst.
Cerium(III) oxide in ceramic form, together with Tin(II) oxide (SnO) is used for illumination with UV light.[18]
References
- ^ Tin and Inorganic Tin Compounds: Concise International Chemical Assessment Document 65, (2005), World Health Organization
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A23. ISBN 978-0-618-94690-7.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0615". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c d e f Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5
- ^ Satya Prakash (2000),Advanced Inorganic Chemistry: V. 1, S. Chand, ISBN 81-219-0263-0
- ^ Arthur Sutcliffe (1930) Practical Chemistry for Advanced Students (1949 Ed.), John Murray - London.
- ^ Braun, Rolf Michael; Hoppe, Rudolf (1978). "The First Oxostannate(II): K2Sn2O3". Angewandte Chemie International Edition in English. 17 (6): 449–450. doi:10.1002/anie.197804491.
- ^ Braun, R. M.; Hoppe, R. (1982). "Über Oxostannate(II). III. K2Sn2O3, Rb2Sn2O3 und Cs2Sn2O3 - ein Vergleich". Zeitschrift für Anorganische und Allgemeine Chemie. 485: 15–22. doi:10.1002/zaac.19824850103.
- ^ R M Braun R Hoppe Z. Naturforsch. (1982), 37B, 688-694
- ^ Bring, T.; Jonson, B.; Kloo, L.; Rosdahl, J; Wallenberg, R. (2007), "Colour development in copper ruby alkali silicate glasses. Part I: The impact of tin oxide, time and temperature", Glass Technology, Eur. J. Glass Science & Technology, Part A, 48 (2): 101–108, ISSN 1753-3546
- ^ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ^ Ramik, R. A.; Organ, R. M.; Mandarino, J. A. (2003). "On Type Romarchite and Hydroromarchite from Boundary Falls, Ontario, and Notes on Other Occurrences". The Canadian Mineralogist. 41 (3): 649–657. doi:10.2113/gscanmin.41.3.649.
- ^ Walsh, Aron; Watson, Graeme W. (2004). "Electronic structures of rocksalt, litharge, and herzenbergite SnO by density functional theory". Physical Review B. 70 (23): 235114. Bibcode:2004PhRvB..70w5114W. doi:10.1103/PhysRevB.70.235114.
- ^ Mei, Antonio B.; Miao, Ludi; Wahila, Matthew J.; Khalsa, Guru; Wang, Zhe; Barone, Matthew; Schreiber, Nathaniel J.; Noskin, Lindsey E.; Paik, Hanjong; Tiwald, Thomas E.; Zheng, Qiye (2019-10-21). "Adsorption-controlled growth and properties of epitaxial SnO films". Physical Review Materials. 3 (10): 105202. Bibcode:2019PhRvM...3j5202M. doi:10.1103/PhysRevMaterials.3.105202. S2CID 208008118.
- ^ Moreno, M. S.; Varela, A.; Otero-Díaz, L. C. (1997). "Cation nonstoichiometry in tin-monoxide-phaseSn1−δOwith tweed microstructure". Physical Review B. 56 (9): 5186–5192. doi:10.1103/PhysRevB.56.5186.
- ^ Science and Technology of Chemiresistor Gas Sensors By Dinesh K. Aswal, Shiv K. Gupta (2006), Nova Publishers, ISBN 1-60021-514-9
- ^ "Red Glass Coloration - A Colorimetric and Structural Study" By Torun Bring. Pub. Vaxjo University.
- ^ Peplinski, D.R.; Wozniak, W.T.; Moser, J.B. (1980). "Spectral Studies of New Luminophors for Dental Porcelain" (PDF). Journal of Dental Research. 59 (9). Jdr.iadrjournals.org: 1501–1506. doi:10.1177/00220345800590090801. PMID 6931128. S2CID 20191368. Retrieved 2012-04-05.[permanent dead link]
