Iridium(V) fluoride
Appearance
| |

| |
| Names | |
|---|---|
| Other names
Iridium pentafluoride
| |
| Identifiers | |
| Properties | |
| IrF5 | |
| Molar mass | 287.209 g/mol |
| Appearance | yellow solid |
| Melting point | 104.5 °C (220.1 °F; 377.6 K) |
| Related compounds | |
Other cations
|
Rhodium(V) fluoride, Osmium pentafluoride, Platinum(V) fluoride |
Related compounds
|
Iridium(IV) fluoride, Iridium hexafluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
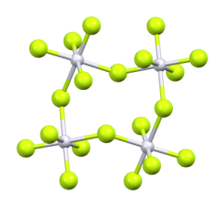
Iridium(V) fluoride, IrF5, is a chemical compound of iridium and fluorine. A highly reactive yellow low melting solid, it has a tetrameric structure, Ir4F20, which contains octahedrally coordinated iridium atoms.[1] This structure is shared with RuF5 and OsF5. It can be prepared by the controlled decomposition of IrF6[1] or the reduction of IrF6 with silicon powder or H2 in anhydrous HF.[2][3]
2 IrF6+H2->2 IrF5+2 HF
See also
References
- ^ a b Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5
- ^ Paine, Robert T.; Asprey, Larned B. (1975). "Reductive syntheses of transition metal fluoride compounds. Synthesis of rhenium, osmium, and iridium pentafluorides and tetrafluorides". Inorganic Chemistry. 14 (5): 1111–1113. doi:10.1021/ic50147a030.
- ^ Neil Bartlett and P. R. Rao (1965). "Iridium pentafluoride". Chem. Commun. (12): 252–253. doi:10.1039/C19650000252.
