Phosphazene
Phosphazenes refer to classes of organophosphorus compounds featuring phosphorus(V) with a double bond between P and N. One class of phosphazenes have the formula RN=P(NR2)3. These phosphazenes are also known as iminophosphoranes and phosphine imides. They are superbases.[1] Another class of compounds called phosphazenes are represented with the formula [X2PN]n, where X = halide, alkoxide, amide. One example is hexachlorocyclotriphosphazene. Bis(triphenylphosphine)iminium chloride is also referred to as a phosphazene. This article focuses on those phosphazenes with the formula RN=P(NR2)3.
Phosphazene bases
Phosphazene bases are strong non-metallic non-ionic and low-nucleophilic bases. They are stronger bases than regular amine or amidine bases. Protonation takes place at a doubly bonded nitrogen atom. Related to phosphazene bases are the Verkade bases, which feature P(III) with three amido substituents and a transannular amine. The pKa's of [tert-Bu(H)N=P(N=PNR2)3)3]+ (R = Me, pyrrolide are 42.7 and 44, respectively. These are the highest pKa measured for the conjugate acid of charge-neutral molecular base.[2]
-
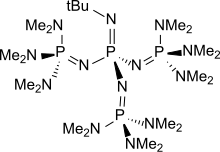
t-Bu-P4
-
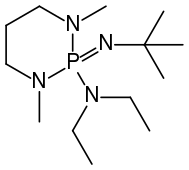
BEMP
Phosphazene bases are established reagents in organic synthesis. Perhaps the best known phosphazene bases are BEMP with an acetonitrile pKa of the conjugate acid of 27.6 and the phosphorimidic triamide t-Bu-P4 (pKBH+ = 42.7) also known as Schwesinger base after one of its inventors.[3]
In one application t-Bu-P4 is employed in a nucleophilic addition converting the pivaldehyde to the alcohol:[4]

The active nucleophile is believed to be a highly reactive phosphazenium species with full negative charge on the arene sp2 carbon.
Besides organic synthesis, phosphazene bases are used as basic titrants in non-aqueous acid-base titration. Their advantages for this are: they are very strong bases in many solvents and their conjugate acids are inert and non-HBD cations.
See also
References
- ^ Superbases for Organic Synthesis: Guanidines, Amidines, Phosphazenes and Related Organocatalysts Tsutomu Ishikawa ISBN 978-0-470-51800-7
- ^ Saame, Jaan; Rodima, Toomas; Tshepelevitsh, Sofja; Kütt, Agnes; Kaljurand, Ivari; Haljasorg, Tõiv; Koppel, Ilmar A.; Leito, Ivo (2016). "Experimental Basicities of Superbasic Phosphonium Ylides and Phosphazenes". The Journal of Organic Chemistry. 81 (17): 7349–7361. doi:10.1021/acs.joc.6b00872. PMID 27392255.
- ^ Schwesinger, Reinhard; Schlemper, Helmut (1987). "Peralkylated Polyaminophosphazenes— Extremely Strong, Neutral Nitrogen Bases". Angewandte Chemie International Edition in English. 26 (11): 1167. doi:10.1002/anie.198711671.
- ^ Suzawa, Koichi; Ueno, Masahiro; Wheatley, Andrew E. H.; Kondo, Yoshinori (2006). "Phosphazene base-promoted functionalization of aryltrimethylsilanes". Chemical Communications (46): 4850. doi:10.1039/b611090h.