Buprenorphine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Subutex, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605002 |
| License data |
|
| Pregnancy category |
|
| Dependence liability | Psychological: High; Physical: Moderate[2] |
| Routes of administration | Under the tongue, through the cheek, IM, transdermal, intranasal, rectally, by mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Sublingual: 30%[5] Intranasal: 48%[6] Buccal: 65%[7][8] |
| Protein binding | 96% |
| Metabolism | Liver (CYP3A4, CYP2C8) |
| Onset of action | Within 30 min[9] |
| Elimination half-life | 37 hours (range 20–70 hours) |
| Duration of action | Up to 24 hrs[9] |
| Excretion | Biliary and kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.052.664 |
| Chemical and physical data | |
| Formula | C29H41NO4 |
| Molar mass | 467.650 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Buprenorphine, sold under the brand name Subutex, among others, is an opioid used to treat opioid use disorder, acute pain, and chronic pain.[9] It can be used under the tongue, in the cheek, by injection, as a skin patch, or as an implant.[9][10] For opioid use disorder, it is typically started when withdrawal symptoms have begun and for the first two days of treatment under direct observation of a health care provider.[9] The combination formulation of buprenorphine/naloxone (Suboxone) is recommended to discourage misuse by injection.[9] Maximum pain relief is generally within an hour with effects up to 24 hours.[9]
Side effects may include respiratory depression (decreased breathing), sleepiness, adrenal insufficiency, QT prolongation, low blood pressure, allergic reactions, and opioid addiction.[9] Among those with a history of seizures, there is a risk of further seizures.[9] Opioid withdrawal following stopping buprenorphine is generally less severe than with other opioids.[9] It is unclear if use during pregnancy is safe and use while breastfeeding is not recommended.[9] Buprenorphine affects different types of opioid receptors in different ways.[9] Depending on the type of receptor it may be an agonist, partial agonist, or antagonist.[9]
Buprenorphine was patented in 1965 and approved for medical use in the United States in 1981.[9][11] In 2017, 14.6 million prescriptions for the medication were written in the United States.[12] It is also a common drug of abuse, being used in place of heroin.[12] Buprenorphine may be used recreationally by injection or in the nose for the high it produces.[12] In the United States, it is a Schedule III controlled substance.[12]
Medical uses
Opioid use disorder

Buprenorphine is used to treat people with opioid use disorder.[9][13]: 84–7 The combination formulation of buprenorphine/naloxone is generally preferred as naloxone, an opioid antagonist, has a higher bioavailability intravenously and results in acute withdrawal if the formulation is crushed and injected.[9][14]: 99 Prior to starting buprenorphine, individuals should wait long enough after their last dose of opioid until they have some withdrawal symptoms to allow for the medication to bind the receptors, but if taken too soon, buprenorphine can displace other opioids bound to the receptors and precipitate an acute withdrawal. The dose of buprenorphine is then adjusted until symptoms improve, and individuals remain on a maintenance dose through treatment.[14]: 99–100 [15]
Buprenorphine versus methadone
Both buprenorphine and methadone are medications used for detoxification and opioid replacement therapy and appear to have similar effectiveness based on limited data[16] and are safe for pregnant women with opioid use disorder,[14]: 101 [15] although preliminary evidence suggests that methadone is more likely to cause neonatal abstinence syndrome.[17] In the US, only designated clinics can prescribe methadone for opioid use disorder in which people starting treatment must follow-up daily, which may be appropriate for those requiring a more structured environment. Alternatively, buprenorphine can be prescribed by any clinician with a waiver allowing people to receive treatment as a part of their routine care.[13]: 84–5
Chronic pain
A transdermal patch is available for the treatment of chronic pain.[9] These patches are not indicated for use in acute pain, pain that is expected to last only for a short period of time, or pain after surgery, nor are they recommended for opioid addiction.[18]
Potency
With respect to equianalgesic dosing, when used sublingually, the potency of buprenorphine is about 40 to 70 times that of morphine.[19][20][21] When used as a transdermal patch, the potency of buprenorphine may be 100–115 times that of morphine.[19][22]
Adverse effects
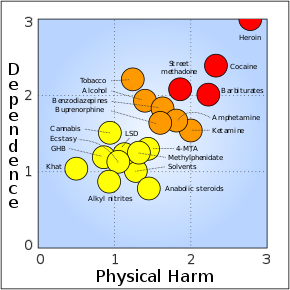
Common adverse drug reactions associated with the use of buprenorphine are similar to those of other opioids and include: nausea and vomiting, drowsiness, dizziness, headache, memory loss, cognitive and neural inhibition, perspiration, itchiness, dry mouth, shrinking of the pupils of the eyes (miosis), orthostatic hypotension, male ejaculatory difficulty, decreased libido, and urinary retention. Constipation and CNS effects are seen less frequently than with morphine.[24]
Respiratory effects
The most severe side effect associated with buprenorphine is respiratory depression (insufficient breathing).[9] It occurs more often in those who are also taking benzodiazepines, alcohol, or have underlying lung disease.[9] The usual reversal agents for opioids, such as naloxone, may be only partially effective and additional efforts to support breathing may be required.[9] Respiratory depression may be less than with other opioids, particularly with chronic use.[15] However, in the setting of acute pain management, buprenorphine appears to cause the same rate of respiratory depression as other opioids such as morphine.[25]
Buprenorphine dependence
Buprenorphine treatment carries the risk of causing psychological or physical dependence. Buprenorphine has a slow onset and a long half-life of 24 to 60 hours. Once a person has stabilized on the medication, there are three options: continual use, switching to buprenorphine/naloxone, or medically supervised withdrawal.[15]
Pain management
It is difficult to achieve acute opioid analgesia in persons using buprenorphine for opioid replacement therapy.[26]
Pharmacology
Pharmacodynamics
| Site | Ki (nM) | Action | Species | Ref |
|---|---|---|---|---|
| MOR | 0.21–1.5 0.081 |
Partial agonist | Human Monkey |
[28][29][30] [31] |
| DOR | 2.9–6.1 0.82 |
Antagonist | Human Monkey |
[28][30][32] [31] |
| KOR | 0.62–2.5 0.44 |
Antagonist | Human Monkey |
[28][30][32] [31] |
| NOP | 77.4 | Partial agonist | Human | [29][30][32] |
| σ1 | >100,000 | ND | ND | [33] |
| σ2 | ND | ND | ND | ND |
| NMDA | ND | ND | ND | ND |
| TLR4 | >10,000 | Agonist | Human | [34] |
| SERT | >100,000 | ND | Rat | [35] |
| NET | >100,000 | ND | Rat | [35] |
| DAT | ND | ND | ND | ND |
| VGSC | 33,000 (IC50) | Inhibitor | Rodent | [36] |
| Values are Ki (nM), unless otherwise noted. The smaller the value, the more strongly the drug binds to the site. | ||||
Opioid receptor modulator
Buprenorphine has been reported to possess the following pharmacological activity:[30]
- μ-Opioid receptor (MOR): Partial agonist. Binds with high affinity, but only partially activates the receptor. This property allows buprenorphine to act similarly to full opioid agonists at lower doses (mainly in non-tolerant individuals), reaching a ceiling/plateau at higher doses after which there is no further increase in typical opioid effects (therapeutic or recreational).[37] This behavior is responsible for buprenorphine's ability to block most MOR agonists and the phenomenon of precipitated withdrawal when used in actively opioid dependent persons.
- κ-Opioid receptor (KOR): Antagonist.[38]
- δ-Opioid receptor (DOR): Antagonist.[38]
- Nociceptin receptor (NOP, ORL-1): Weak affinity. Very weak partial agonist.
In simplified terms, buprenorphine can essentially be thought of as a non-selective, mixed agonist–antagonist opioid receptor modulator,[39] acting as a weak partial agonist of the MOR, an antagonist of the KOR, an antagonist of the DOR, and a relatively low-affinity, very weak partial agonist of the ORL-1.[32][40][41][42][43][44]
Although buprenorphine is a partial agonist of the MOR, human studies have found that it acts like a full agonist with respect to analgesia in non-opioid-tolerant individuals.[45] Conversely, buprenorphine behaves like a partial agonist of the MOR with respect to respiratory depression.[45]
Buprenorphine is also known to bind to with high affinity and antagonize the putative ε-opioid receptor.[46][47]
Full analgesic efficacy of buprenorphine requires both exon 11-[48] and exon 1-associated μ-opioid receptor splice variants.[49]
The active metabolites of buprenorphine are not thought to be clinically important in its central nervous system effects.[45]
Other actions
Unlike some other opioids and opioid antagonists, buprenorphine binds only weakly to and possesses little if any activity at the sigma receptor.[50][51]
Buprenorphine also blocks voltage-gated sodium channels via the local anesthetic binding site, and this underlies its potent local anesthetic properties.[36]
Similarly to various other opioids, buprenorphine has also been found to act as an agonist of the toll-like receptor 4, albeit with very low affinity.[34]
Pharmacokinetics
Buprenorphine is metabolized by the liver, via CYP3A4 (also CYP2C8 seems to be involved) isozymes of the cytochrome P450 enzyme system, into norbuprenorphine (by N-dealkylation). The glucuronidation of buprenorphine is primarily carried out by UGT1A1 and UGT2B7, and that of norbuprenorphine by UGT1A1 and UGT1A3. These glucuronides are then eliminated mainly through excretion into bile. The elimination half-life of buprenorphine is 20 to 73 hours (mean 37 hours). Due to the mainly hepatic elimination, there is no risk of accumulation in people with renal impairment.[52]
One of the major active metabolites of buprenorphine is norbuprenorphine, which, in contrast to buprenorphine itself, is a full agonist of the MOR, DOR, and ORL-1, and a partial agonist at the KOR.[53][54] However, relative to buprenorphine, norbuprenorphine has extremely little antinociceptive potency (1/50th that of buprenorphine), but markedly depresses respiration (10-fold more than buprenorphine).[55] This may be explained by very poor brain penetration of norbuprenorphine due to a high affinity of the compound for P-glycoprotein.[55] In contrast to norbuprenorphine, buprenorphine and its glucuronide metabolites are negligibly transported by P-glycoprotein.[55]
The glucuronides of buprenorphine and norbuprenorphine are also biologically active, and represent major active metabolites of buprenorphine.[56] Buprenorphine-3-glucuronide has affinity for the MOR (Ki = 4.9 pM), DOR (Ki = 270 nM) and ORL-1 (Ki = 36 μM), and no affinity for the KOR. It has a small antinociceptive effect and no effect on respiration. Norbuprenorphine-3-glucuronide has no affinity for the MOR or DOR, but does bind to the KOR (Ki = 300 nM) and ORL-1 (Ki = 18 μM). It has a sedative effect but no effect on respiration.
Chemistry
Buprenorphine is a semi-synthetic analogue of thebaine[57] and is fairly soluble in water, as its hydrochloride salt.[58] It degrades in the presence of light.[58]
Detection in body fluids
Buprenorphine and norbuprenorphine may be quantitated in blood or urine to monitor use or abuse, confirm a diagnosis of poisoning, or assist in a medicolegal investigation. There is a significant overlap of drug concentrations in body fluids within the possible spectrum of physiological reactions ranging from asymptomatic to comatose. Therefore, it is critical to have knowledge of both the route of administration of the drug and the level of tolerance to opioids of the individual when results are interpreted.[59]
History
In 1969, researchers at Reckitt & Colman (now Reckitt Benckiser) had spent 10 years attempting to synthesize an opioid compound "with structures substantially more complex than morphine [that] could retain the desirable actions whilst shedding the undesirable side effects". Physical dependence and withdrawal from buprenorphine itself remain important issues since buprenorphine is a long-acting opioid.[60] Reckitt found success when researchers synthesized RX6029 which had showed success in reducing dependence in test animals. RX6029 was named buprenorphine and began trials on humans in 1971.[61][62] By 1978, buprenorphine was first launched in the UK as an injection to treat severe pain, with a sublingual formulation released in 1982.
Society and culture
Regulation
United States
In the United States, buprenorphine and buprenorphine with naloxone were approved for opioid use disorder by the United States Food and Drug Administration in October 2002.[63] The DEA rescheduled buprenorphine from a Schedule V drug to a Schedule III drug just before approval.[64] The ACSCN for buprenorphine is 9064, and being a Schedule III substance it does not have an annual manufacturing quota imposed by the DEA.[65] The salt in use is the hydrochloride, which has a free base conversion ratio of 0.928.
In the years prior to buprenorphine/naloxone's approval, Reckitt Benckiser had lobbied Congress to help craft the Drug Addiction Treatment Act of 2000 (DATA 2000), which gave authority to the Secretary of Health and Human Services to grant a waiver to physicians with certain training to prescribe and administer Schedule III, IV, or V narcotic drugs for the treatment of addiction or detoxification. Prior to the passage of this law, such treatment was not permitted in outpatient settings except for clinics designed specifically for drug addiction.[66]
The waiver, which can be granted after the completion of an eight-hour course, is required for outpatient treatment of opioid addiction with buprenorphine. Initially, the number of people each approved physician could treat was limited to ten. This was eventually modified to allow approved physicians to treat up to a hundred people with buprenorphine for opioid addiction in an outpatient setting.[67] This limit was increased by the Obama administration, raising the number of patients to which doctors can prescribe to 275.[68]
New Jersey authorized paramedics to give buprenorphine to people at the scene after they have recovered from an overdose.[69]
Europe
In the European Union, Subutex and Suboxone, buprenorphine's high-dose sublingual tablet preparations, were approved for opioid use disorder treatment in September 2006.[70] In the Netherlands, buprenorphine is a List II drug of the Opium Law, though special rules and guidelines apply to its prescription and dispensation.
Brand names
Buprenorphine is available under the trade names Cizdol, Suboxone (with naloxone), Subutex (typically used for opioid use disorder), Zubsolv, Bunavail, Sublocade (monthly injection, approved in the US in 2018),[71][72][73] Probuphine, Temgesic (sublingual tablets for moderate to severe pain), Buprenex (solutions for injection often used for acute pain in primary-care settings), Norspan and Butrans (transdermal preparations used for chronic pain).[58]
Buprenorphine has been introduced in most European countries as a transdermal formulation (marketed as Transtec) for the treatment of chronic pain not responding to non-opioids.
Veterinary medicine
It has veterinary medical use for treatment of pain in dogs and cats.[74][75][76][1]
Research
Depression
Some evidence supports the use of buprenorphine for depression.[77] Buprenorphine/samidorphan, a combination product of buprenorphine and samidorphan (a preferential μ-opioid receptor antagonist), appears useful for treatment-resistant depression.[78]
Cocaine dependence
In combination with samidorphan or naltrexone (μ-opioid receptor antagonists), buprenorphine is under investigation for the treatment of cocaine dependence, and recently demonstrated effectiveness for this indication in a large-scale (n = 302) clinical trial (at a high buprenorphine dose of 16 mg but not a low dose of 4 mg).[79][80]
Neonatal abstinence
Buprenorphine has been used in the treatment of the neonatal abstinence syndrome,[81] a condition in which newborns exposed to opioids during pregnancy demonstrate signs of withdrawal.[82] In the United States, use currently is limited to infants enrolled in a clinical trial conducted under an FDA approved investigational new drug (IND) application.[83] Preliminary research suggests that buprenorphine is associated with shorter time in hospital for neonates, compared to methadone.[84] An ethanolic formulation used in neonates is stable at room temperature for at least 30 days.[85]
Obsessive–compulsive disorder
In one study, buprenorphine was found to be effective in a subset of individuals with treatment-refractory obsessive–compulsive disorder.[86]
References
- ^ a b "Buprenorphine Use During Pregnancy". Drugs.com. 14 October 2019. Retrieved 17 May 2020.
- ^ Bonewit-West, Kathy; Hunt, Sue A.; Applegate, Edith (2012). Today's Medical Assistant: Clinical and Administrative Procedures. Elsevier Health Sciences. p. 571. ISBN 9781455701506.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Health Canada (6 December 2004). "ARCHIVED - Report Stakeholder Workshop on a National Buprenorphine Program". aem. Retrieved 10 January 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ Mendelson J, Upton RA, Everhart ET, Jacob P, Jones RT (January 1997). "Bioavailability of sublingual buprenorphine". Journal of Clinical Pharmacology. 37 (1): 31–7. doi:10.1177/009127009703700106. PMID 9048270.
- ^ Eriksen J, Jensen NH, Kamp-Jensen M, Bjarnø H, Friis P, Brewster D (November 1989). "The systemic availability of buprenorphine administered by nasal spray". The Journal of Pharmacy and Pharmacology. 41 (11): 803–5. doi:10.1111/j.2042-7158.1989.tb06374.x. PMID 2576057.
- ^ "Buprenorphine / Naloxone Buccal Film (BUNAVAIL) C-III" (PDF). Pharmacy Benefits Management (PBM) Services. September 2014.
{{cite web}}: CS1 maint: url-status (link) - ^ BUNAVAIL (buprenorphine and naloxone) buccal film, CIII [prescribing information online]. BioDelivery BioDelivery Sciences International, Inc. (BDSI), Raleigh, NC. Jun 2014.
- ^ a b c d e f g h i j k l m n o p q r s t "Buprenorphine Hydrochloride". drugs.com. American Society of Health-System Pharmacists. 26 January 2017. Retrieved 17 March 2017.
- ^ "Press Announcements – FDA approves first buprenorphine implant for treatment of opioid dependence". FDA. Retrieved 12 December 2017.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 528. ISBN 9783527607495.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b c d Drug Enforcement Administration (July 2019). "Buprenorphine" (PDF). DEA. Retrieved 3 December 2017.
{{cite web}}: CS1 maint: url-status (link) - ^ a b Levounis, Petros; Avery, Jonathan (2018). "Patient Assessment". In Renner, John A., Jr.; Levounis, Petros; LaRose, Anna T. (eds.). Office-based buprenorphine treatment of opioid use disorder. Arlington, VA: American Psychiatric Association Publishing. ISBN 978-1-61537-170-9. OCLC 1002302926.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)CS1 maint: multiple names: editors list (link) - ^ a b c Restrepo, Richard; Levounis, Petros (2018). "Clinical Use of Buprenorphine". In Renner, John A., Jr.; Levounis, Petros; LaRose, Anna T. (eds.). Office-based buprenorphine treatment of opioid use disorder. Arlington, VA: American Psychiatric Association Publishing. ISBN 978-1-61537-170-9. OCLC 1002302926.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)CS1 maint: multiple names: editors list (link) - ^ a b c d "Buprenorphine". www.samhsa.gov. 31 May 2016. Retrieved 3 December 2017.
- ^ Gowing L, Ali R, White JM, Mbewe D (February 2017). "Buprenorphine for managing opioid withdrawal". The Cochrane Database of Systematic Reviews. 2: CD002025. doi:10.1002/14651858.CD002025.pub5. PMC 6464315. PMID 28220474.
- ^ Lemon LS, Caritis SN, Venkataramanan R, Platt RW, Bodnar LM (March 2018). "Methadone Versus Buprenorphine for Opioid Use Dependence and Risk of Neonatal Abstinence Syndrome". Epidemiology. 29 (2): 261–268. doi:10.1097/EDE.0000000000000780. PMC 5792296. PMID 29112519.
Methadone is associated with increased risk of neonatal abstinence syndrome compared with buprenorphine in infants exposed in utero. This association is subject to minimal bias due to unmeasured confounding by severity of addiction.
- ^ "Butrans Medication Guide". Butrans Medication Guide. Purdue Pharma L.P. Retrieved 7 July 2014.
- ^ a b Cote J, Montgomery L (July 2014). "Sublingual buprenorphine as an analgesic in chronic pain: a systematic review". Pain Medicine. 15 (7): 1171–8. doi:10.1111/pme.12386. PMID 24995716.
- ^ "Ch. 4 Narcotics: Narcotics Treatment Drugs: Buprenorphine". Drugs of Abuse. Drug Enforcement Administration, U.S. Department of Justice. 2005. Archived from the original on 2 November 2006.
- ^ "Opioid Conversion Guide" (PDF). Department of Health, Government of Western Australia. February 2016.
{{cite web}}: CS1 maint: url-status (link) - ^ Khanna IK, Pillarisetti S (2015). "Buprenorphine - an attractive opioid with underutilized potential in treatment of chronic pain". Journal of Pain Research. 8: 859–70. doi:10.2147/JPR.S85951. PMC 4675640. PMID 26672499.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Nutt D, King LA, Saulsbury W, Blakemore C (March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". Lancet. 369 (9566): 1047–53. doi:10.1016/S0140-6736(07)60464-4. PMID 17382831.
- ^ Budd K, Raffa RB. (eds.) Buprenorphine – The unique opioid analgesic. Thieme, 200, ISBN 3-13-134211-0
- ^ White LD, Hodge A, Vlok R, Hurtado G, Eastern K, Melhuish TM (April 2018). "Efficacy and adverse effects of buprenorphine in acute pain management: systematic review and meta-analysis of randomised controlled trials". British Journal of Anaesthesia. 120 (4): 668–678. doi:10.1016/j.bja.2017.11.086. PMID 29576108.
- ^ Alford DP, Compton P, Samet JH (January 2006). "Acute pain management for patients receiving maintenance methadone or buprenorphine therapy". Annals of Internal Medicine. 144 (2): 127–34. doi:10.7326/0003-4819-144-2-200601170-00010. PMC 1892816. PMID 16418412.
- ^ Roth BL, Driscol J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- ^ a b c Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, et al. (March 1998). "Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications". NIDA Research Monograph. 178: 440–66. PMID 9686407.
- ^ a b Khroyan TV, Polgar WE, Jiang F, Zaveri NT, Toll L (December 2009). "Nociceptin/orphanin FQ receptor activation attenuates antinociception induced by mixed nociceptin/orphanin FQ/mu-opioid receptor agonists". The Journal of Pharmacology and Experimental Therapeutics. 331 (3): 946–53. doi:10.1124/jpet.109.156711. PMC 2784721. PMID 19713488.
- ^ a b c d e Khroyan TV, Wu J, Polgar WE, Cami-Kobeci G, Fotaki N, Husbands SM, Toll L (January 2015). "BU08073 a buprenorphine analogue with partial agonist activity at μ-receptors in vitro but long-lasting opioid antagonist activity in vivo in mice". British Journal of Pharmacology. 172 (2): 668–80. doi:10.1111/bph.12796. PMC 4292977. PMID 24903063.
- ^ a b c Negus SS, Bidlack JM, Mello NK, Furness MS, Rice KC, Brandt MR (November 2002). "Delta opioid antagonist effects of buprenorphine in rhesus monkeys". Behavioural Pharmacology. 13 (7): 557–70. doi:10.1097/00008877-200211000-00005. PMID 12409994.
- ^ a b c d Lutfy K, Cowan A (October 2004). "Buprenorphine: a unique drug with complex pharmacology". Current Neuropharmacology. 2 (4): 395–402. doi:10.2174/1570159043359477. PMC 2581407. PMID 18997874.
- ^ Freye, Enno (1987). "Interaction of Mixed Agonist-Antagonists with Different Receptor Sites Using Nalbuphine as a Model Substance". Opioid Agonists, Antagonists and Mixed Narcotic Analgesics. pp. 67–78. doi:10.1007/978-3-642-71854-0_6. ISBN 978-3-540-17471-4.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, et al. (January 2010). "Evidence that opioids may have toll-like receptor 4 and MD-2 effects". Brain, Behavior, and Immunity. 24 (1): 83–95. doi:10.1016/j.bbi.2009.08.004. PMC 2788078. PMID 19679181.
- ^ a b Codd EE, Shank RP, Schupsky JJ, Raffa RB (September 1995). "Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: structural determinants and role in antinociception". The Journal of Pharmacology and Experimental Therapeutics. 274 (3): 1263–70. PMID 7562497.
- ^ a b Leffler A, Frank G, Kistner K, Niedermirtl F, Koppert W, Reeh PW, Nau C (June 2012). "Local anesthetic-like inhibition of voltage-gated Na(+) channels by the partial μ-opioid receptor agonist buprenorphine". Anesthesiology. 116 (6): 1335–46. doi:10.1097/ALN.0b013e3182557917. PMID 22504149.
- ^ Lutfy, K., & Cowan, A. (2004). Buprenorphine: A Unique Drug with Complex Pharmacology. Current Neuropharmacology, 2(4), 395–402. doi:10.2174/1570159043359477
- ^ a b Benzon, Honorio; Raja, Srinivasa N.; Fishman, Scott M.; Liu, Spencer S.; Cohen, Steven P. (2017). Essentials of Pain Medicine E-Book. Elsevier Health Sciences. p. 382. ISBN 9780323445412.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Jacob JJ, Michaud GM, Tremblay EC (1979). "Mixed agonist-antagonist opiates and physical dependence". British Journal of Clinical Pharmacology. 7 Suppl 3: 291S–296S. doi:10.1111/j.1365-2125.1979.tb04703.x. PMC 1429306. PMID 572694.
- ^ Kress HG (March 2009). "Clinical update on the pharmacology, efficacy and safety of transdermal buprenorphine". European Journal of Pain. 13 (3): 219–30. doi:10.1016/j.ejpain.2008.04.011. PMID 18567516.
- ^ Robinson SE (2002). "Buprenorphine: an analgesic with an expanding role in the treatment of opioid addiction". CNS Drug Reviews. 8 (4): 377–90. doi:10.1111/j.1527-3458.2002.tb00235.x. PMC 6741692. PMID 12481193.
- ^ Ruiz, Pedro; Strain, Eric C. (2011). Lowinson and Ruiz's Substance Abuse: A Comprehensive Textbook. Lippincott Williams & Wilkins. p. 439. ISBN 978-1-60547-277-5.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Bidlack JM (2014). Mixed kappa/mu partial opioid agonists as potential treatments for cocaine dependence. Advances in Pharmacology. Vol. 69. pp. 387–418. doi:10.1016/B978-0-12-420118-7.00010-X. ISBN 9780124201187. PMID 24484983.
{{cite book}}:|journal=ignored (help) - ^ Ehrich E, Turncliff R, Du Y, Leigh-Pemberton R, Fernandez E, Jones R, Fava M (May 2015). "Evaluation of opioid modulation in major depressive disorder". Neuropsychopharmacology. 40 (6): 1448–55. doi:10.1038/npp.2014.330. PMC 4397403. PMID 25518754.
- ^ a b c Coller JK, Christrup LL, Somogyi AA (February 2009). "Role of active metabolites in the use of opioids". European Journal of Clinical Pharmacology. 65 (2): 121–39. doi:10.1007/s00228-008-0570-y. PMID 18958460.
- ^ Mizoguchi H, Wu HE, Narita M, Hall FS, Sora I, Uhl GR, et al. (2002). "Antagonistic property of buprenorphine for putative epsilon-opioid receptor-mediated G-protein activation by beta-endorphin in pons/medulla of the mu-opioid receptor knockout mouse". Neuroscience. 115 (3): 715–21. doi:10.1016/s0306-4522(02)00486-4. PMID 12435410.
- ^ Mizoguchi H, Spaulding A, Leitermann R, Wu HE, Nagase H, Tseng LF (July 2003). "Buprenorphine blocks epsilon- and micro-opioid receptor-mediated antinociception in the mouse". The Journal of Pharmacology and Experimental Therapeutics. 306 (1): 394–400. doi:10.1124/jpet.103.048835. PMID 12721333. S2CID 44036890.
- ^ Xu J, Xu M, Hurd YL, Pasternak GW, Pan YX (February 2009). "Isolation and characterization of new exon 11-associated N-terminal splice variants of the human mu opioid receptor gene". Journal of Neurochemistry. 108 (4): 962–72. doi:10.1111/j.1471-4159.2008.05833.x. PMC 2727151. PMID 19077058.
- ^ Grinnell S et al. (2014): Buprenorphine analgesia requires exon 11-associated mu opioid receptor splice variants. The FASEB Journal
- ^ Doweiko, Harold E. (14 March 2014). Concepts of Chemical Dependency. Cengage Learning. pp. 149–. ISBN 978-1-285-45717-8.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ USP DI. United States Pharmacopeial Convention. 1997. ISBN 9780913595947.
- ^ Moody DE, Fang WB, Lin SN, Weyant DM, Strom SC, Omiecinski CJ (December 2009). "Effect of rifampin and nelfinavir on the metabolism of methadone and buprenorphine in primary cultures of human hepatocytes". Drug Metabolism and Disposition. 37 (12): 2323–9. doi:10.1124/dmd.109.028605. PMC 2784702. PMID 19773542.
- ^ Yassen A, Kan J, Olofsen E, Suidgeest E, Dahan A, Danhof M (May 2007). "Pharmacokinetic-pharmacodynamic modeling of the respiratory depressant effect of norbuprenorphine in rats". The Journal of Pharmacology and Experimental Therapeutics. 321 (2): 598–607. doi:10.1124/jpet.106.115972. PMID 17283225.
- ^ Huang P, Kehner GB, Cowan A, Liu-Chen LY (May 2001). "Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist". The Journal of Pharmacology and Experimental Therapeutics. 297 (2): 688–95. PMID 11303059.
- ^ a b c Brown SM, Campbell SD, Crafford A, Regina KJ, Holtzman MJ, Kharasch ED (October 2012). "P-glycoprotein is a major determinant of norbuprenorphine brain exposure and antinociception". The Journal of Pharmacology and Experimental Therapeutics. 343 (1): 53–61. doi:10.1124/jpet.112.193433. PMC 3464040. PMID 22739506.
- ^ Brown SM, Holtzman M, Kim T, Kharasch ED (December 2011). "Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active". Anesthesiology. 115 (6): 1251–60. doi:10.1097/ALN.0b013e318238fea0. PMC 3560935. PMID 22037640.
- ^ Heel RC, Brogden RN, Speight TM, Avery GS (February 1979). "Buprenorphine: a review of its pharmacological properties and therapeutic efficacy". Drugs. 17 (2): 81–110. doi:10.2165/00003495-197917020-00001. PMID 378645.
- ^ a b c "Buprenorphine". Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. 14 January 2014. Retrieved 6 April 2014.
- ^ Baselt R (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. pp. 190–192. ISBN 978-0962652370.
- ^ "IMPORTANT SAFETY INFORMATION". Archived from the original on 18 March 2019. Retrieved 25 June 2016.
- ^ Campbell ND, Lovell AM (February 2012). "The history of the development of buprenorphine as an addiction therapeutic". Annals of the New York Academy of Sciences. 1248 (1): 124–39. Bibcode:2012NYASA1248..124C. doi:10.1111/j.1749-6632.2011.06352.x. PMID 22256949.
- ^ Louis S. Harris, ed. (1998). Problems of Drug Dependence, 1998: Proceedings of the 66th Annual Scientific Meeting, The College on Problems of Drug Dependence, Inc (PDF). NIDA Research Monograph 179. Archived from the original (PDF) on 3 December 2016. Retrieved 5 August 2012.
- ^ Subutex and Suboxone Approval Letter. U.S. Food and Drug Administration (8 October 2002). fda.gov.
- ^ 67 FR 62354, 7 October 2002
- ^ Quotas – Conversion Factors for Controlled Substances. Deadiversion.usdoj.gov. Retrieved on 7 November 2016.
- ^ "Drug Addiction Treatment Act of 2000" Archived 4 March 2013 at the Wayback Machine. SAMHSA, U.S. Department of Health & Human Services.
- ^ The National Alliance of Advocates for Buprenorphine Treatment. naabt.org. Retrieved on 19 May 2013.
- ^ Obama administration's change on buprenorphine policy. Business Insider (6 July 2016). Retrieved on 2016-11-07.
- ^ "In national first, N.J. program will let paramedics administer buprenorphine". STAT. 26 June 2019. Retrieved 21 November 2019.
- ^ Suboxone EU Approval. Ema.europa.eu. Retrieved on 7 November 2016.
- ^ "Press Announcements – FDA approves first once-monthly buprenorphine injection, a medication-assisted treatment option for opioid use disorder". www.fda.gov. Retrieved 5 December 2017.
- ^ "Indivior drug to fight opioid addiction approved by U.S. FDA". Reuters. 2017. Retrieved 5 December 2017.
- ^ "Sublocade Now Available for Moderate-to-Severe Opioid Use Disorder". MPR. 1 March 2018. Retrieved 23 September 2019.
- ^ Claude, Andrew (June 2015). "Buprenorphine" (PDF). cliniciansbrief.com. Archived from the original (PDF) on 16 May 2017. Retrieved 25 February 2017.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Kukanich B, Papich MG (14 May 2013). "Opioid Analgesic Drugs". In Riviere JE, Papich MG (eds.). Veterinary Pharmacology and Therapeutics (9 ed.). John Wiley & Sons. pp. 323–325. ISBN 9781118685907.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Steagall PV, Ruel HL, Yasuda T, Monteiro BP, Watanabe R, Evangelista MC, Beaudry F (May 2020). "Pharmacokinetics and analgesic effects of intravenous, intramuscular or subcutaneous buprenorphine in dogs undergoing ovariohysterectomy: a randomized, prospective, masked, clinical trial". BMC Veterinary Research. 16 (1): 154. doi:10.1186/s12917-020-02364-w. PMC 7245774. PMID 32448336.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Stanciu CN, Glass OM, Penders TM (April 2017). "Use of Buprenorphine in treatment of refractory depression-A review of current literature". Asian Journal of Psychiatry. 26: 94–98. doi:10.1016/j.ajp.2017.01.015. PMID 28483102.
- ^ Ragguett RM, Rong C, Rosenblat JD, Ho RC, McIntyre RS (April 2018). "Pharmacodynamic and pharmacokinetic evaluation of buprenorphine + samidorphan for the treatment of major depressive disorder". Expert Opinion on Drug Metabolism & Toxicology. 14 (4): 475–482. doi:10.1080/17425255.2018.1459564. PMID 29621905.
- ^ Ling W, Hillhouse MP, Saxon AJ, Mooney LJ, Thomas CM, Ang A, et al. (August 2016). "Buprenorphine + naloxone plus naltrexone for the treatment of cocaine dependence: the Cocaine Use Reduction with Buprenorphine (CURB) study". Addiction. 111 (8): 1416–27. doi:10.1111/add.13375. PMC 4940267. PMID 26948856.
- ^ "Alkermes Presents Positive Clinical Data of ALKS 5461 at 52nd Annual New Clinical Drug Evaluation Unit Meeting". Reuters. 2012.
- ^ Kraft WK, Gibson E, Dysart K, Damle VS, Larusso JL, Greenspan JS, et al. (September 2008). "Sublingual buprenorphine for treatment of neonatal abstinence syndrome: a randomized trial". Pediatrics. 122 (3): e601-7. doi:10.1542/peds.2008-0571. PMC 2574639. PMID 18694901.
- ^ Kraft WK, van den Anker JN (October 2012). "Pharmacologic management of the opioid neonatal abstinence syndrome". Pediatric Clinics of North America. 59 (5): 1147–65. doi:10.1016/j.pcl.2012.07.006. PMC 4709246. PMID 23036249.
- ^ Clinical trial number NCT00521248 for "Buprenorphine for the Treatment of Neonatal Abstinence Syndrome" at ClinicalTrials.gov
- ^ Tran TH, Griffin BL, Stone RH, Vest KM, Todd TJ (July 2017). "Methadone, Buprenorphine, and Naltrexone for the Treatment of Opioid Use Disorder in Pregnant Women". Pharmacotherapy. 37 (7): 824–839. doi:10.1002/phar.1958. PMID 28543191.
Currently, methadone and buprenorphine are both widely used as the backbone of MAT [medication-assisted treatment]. The distinguishing outcomes in studies among these two opioid agonists are that infants exposed to buprenorphine in clinical trials required shorter treatment duration, less medication to treat the NAS symptoms and experienced shorter hospitalizations compared to infants exposed to methadone. A caveat to these findings is that some of the supporting data were based on using buprenorphine in combination with naloxone instead of buprenorphine as a single agent.
- ^ Anagnostis EA, Sadaka RE, Sailor LA, Moody DE, Dysart KC, Kraft WK (October 2011). "Formulation of buprenorphine for sublingual use in neonates". The Journal of Pediatric Pharmacology and Therapeutics. 16 (4): 281–4. doi:10.5863/1551-6776-16.4.281 (inactive 29 May 2020). PMC 3385042. PMID 22768012.
{{cite journal}}: CS1 maint: DOI inactive as of May 2020 (link) - ^ Liddell MB, Aziz V, Briggs P, Kanakkehewa N, Rawi O (February 2013). "Buprenorphine augmentation in the treatment of refractory obsessive-compulsive disorder". Therapeutic Advances in Psychopharmacology. 3 (1): 15–9. doi:10.1177/2045125312462233. PMC 3736962. PMID 23983988.
External links
- U.S. Federal government buprenorphine program for opioid addiction
- Australian national buprenorphine policy
- "The bitter pill": A Wired magazine article on Suboxone
- "Subu Must Die – How a nation of junkies went cold turkey": A New Republic article on Subutex abuse in the nation of Georgia
- "Buprenorphine". Drug Information Portal. U.S. National Library of Medicine.
- CS1 maint: DOI inactive as of May 2020
- Tertiary alcohols
- Cat medications
- Dog medications
- Schering-Plough brands
- Merck & Co. brands
- Delta-opioid antagonists
- Drug rehabilitation
- Ethers
- Euphoriants
- Kappa antagonists
- Morphinans
- Mu-opioid agonists
- Nociceptin receptor agonists
- Nociceptin receptor antagonists
- Oripavines
- Phenols
- Semisynthetic opioids
- Sodium channel blockers
- RTT
