Butane: Difference between revisions
m Reverted edit(s) by 209.34.127.134 identified as test/vandalism using STiki |
|||
| Line 90: | Line 90: | ||
== Reactions == |
== Reactions == |
||
{{stack| |
{{stack| |
||
[[Image:Spectrum of |
|||
[[Image:Spectrum of blue flame.png|thumb|Spectrum of the blue flame from a [[butane torch]] showing molecular [[Radical (chemistry)|radical]] band emission and [[Swan band]]s]] |
|||
}} |
|||
When oxygen is plentiful, butane burns to form [[carbon dioxide]] and water vapor; when oxygen is limited, carbon (soot) or carbon monoxide may also be formed. |
|||
: 2 C<sub>4</sub>H<sub>10</sub> + 13 O<sub>2</sub> → 8 CO<sub>2</sub> + 10 H<sub>2</sub>O |
|||
The maximum [[adiabatic flame]] temperature of butane with [[air]] is {{convert|2243|K}}. |
|||
''n''-Butane is the feedstock for [[DuPont]]'s catalytic process for the preparation of [[maleic anhydride]]: |
|||
:2 CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub> + 7 O<sub>2</sub> → 2 C<sub>2</sub>H<sub>2</sub>(CO)<sub>2</sub>O + 8 H<sub>2</sub>O |
|||
''n''-Butane, like all hydrocarbons, undergoes [[free radical]] chlorination providing both 1-chloro- and 2-chlorobutanes, as well as more highly chlorinated derivatives. The relative rates of the chlorination is partially explained by the differing [[bond dissociation energies]], 425 and 411 [[joule|kJ]]/mol for the two types of C-H bonds. The two central carbon atoms have the slightly weaker C-H bonds.<!--I hope this is right--> |
|||
== Uses == |
== Uses == |
||
Revision as of 23:46, 21 March 2011
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
butane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.136 | ||
| E number | E943a (glazing agents, ...) | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| UN number | 1011 As Liquefied petroleum gas: 1075 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H10 | |||
| Molar mass | 58.124 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Density | 2.48 kg/m3, gas (15 °C, 1 atm) 600 kg/m3, liquid (0 °C, 1 atm) | ||
| Melting point | −138.4 °C (135.4 K) | ||
| Boiling point | −0.5 °C (272.6 K) | ||
| 6.1 mg/100 ml (20 °C) | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −60 °C | ||
| Explosive limits | 1.8 – 8.4% [1] | ||
| Related compounds | |||
| Supplementary data page | |||
| Butane (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Butane is a gas with the formula C4H10 that is an alkane with four carbon atoms. The term may refer to any of two structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, butane refers only to the unbranched n-butane isomer; the other one being called "methylpropane" or isobutane.
Butanes are highly flammable, colorless, easily liquefied gases. The name butane comes from the roots but- (from butyric acid) and -ane.
Isomers
| Common name | normal butane unbranched butane n-butane |
isobutane i-butane |
| IUPAC name | butane | methylpropane |
| Molecular diagram |
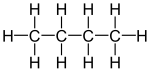
|
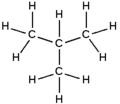
|
| Skeletal diagram |

|
Rotation about the central C-C bond produces two different conformations (trans and gauche) for n-butane.[2]
Reactions
{{stack| [[Image:Spectrum of
Uses

Butane gas is sold bottled as a fuel for cooking and camping. When blended with propane and other hydrocarbons, it is referred to commercially as LPG. It is also used as a petrol component, as a feedstock for the production of base petrochemicals in steam cracking, as fuel for cigarette lighters and as a propellant in aerosol sprays such as deodorants.
Very pure forms of butane, especially isobutane, can be used as refrigerants and have largely replaced the ozone layer-depleting halomethanes, for instance in household refrigerators and freezers. The system operating pressure for butane is lower than for the halomethanes, such as R-12, so R-12 systems such as in automotive air conditioning systems, when converted to butane will not function optimally.
Effects and health issues

Inhalation of butane can cause euphoria, drowsiness, narcosis, asphyxia, cardiac arrhythmia, and frostbite, which can result in death from asphyxiation and ventricular fibrillation. Butane is the most commonly misused volatile substance in the UK, and was the cause of 52% of "solvent related" deaths in 2000.[3] By spraying butane directly into the throat, the jet of fluid can cool rapidly to −20 °C by expansion, causing prolonged laryngospasm.[4] "Sudden sniffer's death" syndrome, first described by Bass in 1970,[5] is the most common single cause of "solvent related" death, resulting in 55% of known fatal cases.[4]
The paper "Emission of nitrogen dioxide from butane gas heaters and stoves indoors", from the American Journal of Applied Sciences, indicates that nitrogen dioxide, a toxic gas, results from burning butane gas, and represents a human health hazard from home heaters and stoves.
See also
References
- ^ MSDS Butane BOC Gases
- ^ Roman M. Balabin (2009). "Enthalpy Difference between Conformations of Normal Alkanes: Raman Spectroscopy Study of n-Pentane and n-Butane". J. Phys. Chem. A. 113 (6): 1012. doi:10.1021/jp809639s. PMID 19152252.
- ^ Trends in death Associated with Abuse of Volatile Substances 1971–2004 Field-Smith M, Bland JM, Taylor JC, et al., Department of Public Health Sciences. London: St George’s Medical School
- ^ a b Ramsey J, Anderson HR, Bloor K, et al. An introduction to the practice, prevalence and chemical toxicology of volatile substance abuse. Hum Toxicol 1989;8:261–269
- ^ Bass M. Sudden sniffing death. JAMA 1970;212:2075–2079
External links
- International Chemical Safety Card 0232
- NIOSH Pocket Guide to Chemical Hazards
- Template:Ecb
- n-Butane Molecule of the Month
- World LP Gas Association (WLPGA)
- UKLPG Propane and Butane in the UK
- Global BioSciences In-Situ Bioremediation utilizing Butane





