Calusterone
From Wikipedia, the free encyclopedia
This is an old revision of this page, as edited by Vanished user 0x8cSXE0x6 (talk | contribs) at 03:47, 30 November 2016. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 03:47, 30 November 2016 by Vanished user 0x8cSXE0x6 (talk | contribs)
 | |
| Clinical data | |
|---|---|
| Trade names | Methosarb, Riedemil |
| Other names | 7β,17α-Dimethyltestosterone; NSC-88536; U-22550 |
| Routes of administration | Oral |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
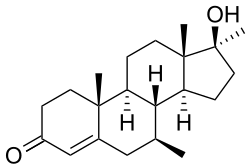
| Formula | C21H32O2 |
| Molar mass | 316.48 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Calusterone (INN, USAN) (brand names Methosarb, Riedemil; former developmental code names NSC-88536, U-22550), also known as 7β,17α-dimethyltestosterone, is an orally active anabolic-androgenic steroid (AAS) that is used as an antineoplastic agent.[1][2] It is a 17α-alkylated AAS similar in structure to bolasterone (which is its 7α-isomer).[1]
References
- ^ a b J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 646–. ISBN 978-1-4757-2085-3.
- ^ I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 52–. ISBN 978-94-011-4439-1.
| ARTooltip Androgen receptor |
| ||||||
|---|---|---|---|---|---|---|---|
| GPRC6A |
| ||||||
This drug article relating to the genito-urinary system is a stub. You can help Wikipedia by expanding it. |
Hidden categories:
