Cannizzaro reaction
The Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction that involves the base-induced disproportionation of an aldehyde lacking a hydrogen atom in the alpha position.[1][2] Cannizzaro first accomplished this transformation in 1853, when he obtained benzyl alcohol and benzoic acid from the treatment of benzaldehyde with potash (potassium carbonate).

The oxidation product is a carboxylic acid and the reduction product is an alcohol. For aldehydes with a hydrogen atom alpha to the carbonyl, i.e. RCHR'CHO, the preferred reaction is an aldol condensation, originating from deprotonation of this hydrogen. Reviews have been published.[3]
Reaction mechanism
The first reaction step is nucleophilic addition of the base (for instance the hydroxide anion) to the carbonyl carbon of the aldehyde. The resulting alkoxide is deprotonated to give a di-anion, known as the Cannizzaro intermediate. Formation of this intermediate requires a strongly basic environment. This reaction is self oxidation-reduction reaction. Alcohols are formed in the result of reduction and salts of carboxylic acid in the result of oxidation.
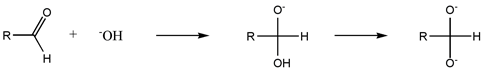
Both intermediates can react further with aldehyde to transfer a hydride, H−. The hydridic character of the C-H is enhanced by the electron-donating character of the alpha oxygen anion. This hydride transfer simultaneously generates an alkoxide anion (RCH2O-) and a carboxylic acid, which is rapidly deprotonated to form the carboxylate. Further evidence for the hydridic character of the Cannizzaro intermediate is provided by the formation of H2 by its reaction with water.

Only aldehydes that cannot form an enolate ion undergo the Cannizzaro reaction. The aldehyde cannot have an enolizable proton. Under the basic conditions that facilitate the reaction, aldehydes that can form an enolate instead undergo aldol condensation. Examples of aldehydes that can undergo a Cannizzaro reaction include formaldehyde and aromatic aldehydes such as benzaldehyde.
Variations
A special condition is the crossed Cannizzaro reaction. This variation is more common these days because the original Cannizzaro reaction yields a mixture of alcohol and carboxylic acid. For example any aldehyde with no alpha hydrogens can be reduced when in the presence of formaldehyde. Formaldehyde is oxidized to formic acid and the corresponding alcohol is obtained in a high yield although the atom economy is still low.
Scope
A solvent-free reaction has been reported involving mixing 2-chlorobenzaldehyde with potassium hydroxide in a mortar and pestle [4]:

References
- ^ Cannizzaro, S. (1853). "Ueber den der Benzoësäure entsprechenden Alkohol". Liebigs Annalen. 88: 129–130. doi:10.1002/jlac.18530880114.
- ^ List, K.; Limpricht, H. (1854). "Ueber das sogenannte Benzoëoxyd und einige andere gepaarte Verbindungen". Liebigs Annalen. 90: 190–210. doi:10.1002/jlac.18540900211.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Geissman, T. A. Org. React. 1944, 2, 94. (Review)
- ^ A Facile Solvent-Free Cannizzaro Reaction Phonchaiya, Sonthi; ; Panijpan, Bhinyo Rajviroongit, Shuleewan; Wright, Tony; Blanchfield, Joanne T. J. Chem. Educ. 2009, 86, 85.
SHOULD BE CORRECTED
