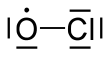Chlorine monoxide
This article includes a list of general references, but it lacks sufficient corresponding inline citations. (April 2020) |
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Chlorine monoxide | |||
| Systematic IUPAC name
Chlorooxidanyl | |||
| Other names
Chlorine(II) oxide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | ClO(.) | ||
| ChEBI | |||
| ChemSpider | |||
| MeSH | Chlorosyl | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| ClO | |||
| Molar mass | 51.45 g·mol−1 | ||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
101.8 kJ/mol[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Chlorine monoxide is a chemical radical with the chemical formula ClO. It plays an important role in the process of ozone depletion. In the stratosphere, chlorine atoms react with ozone molecules to form chlorine monoxide and oxygen.
- Cl· + O
3 → ClO· + O
2
This reaction causes the depletion of the ozone layer.[1] This result ClO· radicals can further react as such:
- ClO· + O· → Cl· + O
2
regenerating the chlorine radical. In this way, the overall reaction for the decomposition of ozone is catalyzed by chlorine, as ultimately chlorine remains unchanged. The overall reaction is:
- O· + O
3 → + 2O
2
This has been a significant impact of the use of CFCs on the upper stratosphere, however many countries have agreed to ban the use of CFCs. The nonreactive nature of CFC's allows them to pass into the stratosphere, where they undergo photo-dissociation to form Cl radicals. These then readily form chlorine monoxide, and this cycle can continue until two radicals react to form dichlorine monoxide, terminating the radical reaction. Because the concentration of CFCs in atmosphere is very low, the probability of a terminating reaction is exceedingly low, meaning each radical can decompose many thousands of molecules of ozone.
Even though the use of CFCs was banned in many countries, CFCs can stay in the atmosphere for about 50-500 years. This cause many chlorine radicals to be produced and hence a significant amount of ozone molecules are decomposed before the chlorine radicals are able to react with chlorine monoxide to form Dichlorine monoxide.
References
- ^ a b Egon Wiberg; Nils Wiberg; Arnold Frederick Holleman (2001). Inorganic chemistry. Academic Press. p. 462. ISBN 0-12-352651-5.