Coal: Difference between revisions
m Reverted edits by 117.216.42.195 (talk) to last version by Pinethicket |
No edit summary |
||
| Line 10: | Line 10: | ||
}} |
}} |
||
[[File:Coal bituminous.jpg|thumb|Bituminous coal]] |
[[File:Coal bituminous.jpg|thumb|Bituminous coal]] |
||
'''Coal''' (from the Old English term ''col'', which has meant "mineral of fossilized carbon" since the 13th century)<ref>{{OEtymD|coal}}</ref> is a [[combustible]] black or brownish-black [[sedimentary rock]] usually occurring in [[stratum|rock strata]] in layers or veins called '''coal beds<!-- redirected term title-->''' or '''coal seams'''. The harder forms, such as [[anthracite|anthracite coal]], can be regarded as [[metamorphic rock]] because of later exposure to elevated temperature and [[pressure]]. Coal is composed primarily of [[carbon]] along with variable quantities of other elements, chiefly [[hydrogen]], [[sulfur]], [[oxygen]], and [[nitrogen]].<ref>{{cite web|last=Blander|first=M|title=Calculations of the Influence of Additives on Coal Combustion Deposits|url=http://www.anl.gov/PCS/acsfuel/preprint%20archive/Files/Volumes/Vol34-2.pdf|publisher=Argonne National Laboratory|accessdate=17 December 2011|page=315}}</ref> |
Hi Will '''Coal''' (from the Old English term ''col'', which has meant "mineral of fossilized carbon" since the 13th century)<ref>{{OEtymD|coal}}</ref> is a [[combustible]] black or brownish-black [[sedimentary rock]] usually occurring in [[stratum|rock strata]] in layers or veins called '''coal beds<!-- redirected term title-->''' or '''coal seams'''. The harder forms, such as [[anthracite|anthracite coal]], can be regarded as [[metamorphic rock]] because of later exposure to elevated temperature and [[pressure]]. Coal is composed primarily of [[carbon]] along with variable quantities of other elements, chiefly [[hydrogen]], [[sulfur]], [[oxygen]], and [[nitrogen]].<ref>{{cite web|last=Blander|first=M|title=Calculations of the Influence of Additives on Coal Combustion Deposits|url=http://www.anl.gov/PCS/acsfuel/preprint%20archive/Files/Volumes/Vol34-2.pdf|publisher=Argonne National Laboratory|accessdate=17 December 2011|page=315}}</ref> |
||
Throughout history, coal has been used as an energy resource, primarily burned for the production of electricity and/or heat, and is also used for industrial purposes, such as refining metals. A [[fossil fuel]], coal forms when dead plant matter is converted into [[peat]], which in turn is converted into [[lignite]], then sub-bituminous coal, after that [[bituminous coal]], and lastly [[anthracite]]. This involves biological and geological processes that take place over a long period. The [[Energy Information Administration]] estimates coal [[Mineral resource classification|reserves]] at {{val|948|e=9}} [[short ton]]s (860 [[Gigatonne|Gt]]).<ref name=EIAreserves/> One estimate for [[Mineral resource classification|resources]] is 18 000 Gt.<ref>{{cite journal|title=Fire in the hole: After fracking comes coal|journal=[[New Scientist]]|date=Feb 15, 2014|pages=36–41|url=http://www.newscientist.com/article/mg22129560.400-fire-in-the-hole-after-fracking-comes-coal.html?full=true|author=Fred Pearce|authorlink=Fred Pearce}}</ref> |
Throughout history, coal has been used as an energy resource, primarily burned for the production of electricity and/or heat, and is also used for industrial purposes, such as refining metals. A [[fossil fuel]], coal forms when dead plant matter is converted into [[peat]], which in turn is converted into [[lignite]], then sub-bituminous coal, after that [[bituminous coal]], and lastly [[anthracite]]. This involves biological and geological processes that take place over a long period. The [[Energy Information Administration]] estimates coal [[Mineral resource classification|reserves]] at {{val|948|e=9}} [[short ton]]s (860 [[Gigatonne|Gt]]).<ref name=EIAreserves/> One estimate for [[Mineral resource classification|resources]] is 18 000 Gt.<ref>{{cite journal|title=Fire in the hole: After fracking comes coal|journal=[[New Scientist]]|date=Feb 15, 2014|pages=36–41|url=http://www.newscientist.com/article/mg22129560.400-fire-in-the-hole-after-fracking-comes-coal.html?full=true|author=Fred Pearce|authorlink=Fred Pearce}}</ref> |
||
Revision as of 21:35, 6 October 2014
| Sedimentary rock | |
 Anthracite coal | |
| Composition | |
|---|---|
| Primary | carbon |
| Secondary | hydrogen, sulfur, oxygen, nitrogen |

Hi Will Coal (from the Old English term col, which has meant "mineral of fossilized carbon" since the 13th century)[1] is a combustible black or brownish-black sedimentary rock usually occurring in rock strata in layers or veins called coal beds or coal seams. The harder forms, such as anthracite coal, can be regarded as metamorphic rock because of later exposure to elevated temperature and pressure. Coal is composed primarily of carbon along with variable quantities of other elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.[2]
Throughout history, coal has been used as an energy resource, primarily burned for the production of electricity and/or heat, and is also used for industrial purposes, such as refining metals. A fossil fuel, coal forms when dead plant matter is converted into peat, which in turn is converted into lignite, then sub-bituminous coal, after that bituminous coal, and lastly anthracite. This involves biological and geological processes that take place over a long period. The Energy Information Administration estimates coal reserves at 948×109 short tons (860 Gt).[3] One estimate for resources is 18 000 Gt.[4]
Coal is the largest source of energy for the generation of electricity worldwide, as well as one of the largest worldwide anthropogenic sources of carbon dioxide releases. In 1999, world gross carbon dioxide emissions from coal usage were 8,666 million tonnes of carbon dioxide.[5] In 2011, world gross emissions from coal usage were 14,416 million tonnes.[6] Coal-fired electric power generation emits around 2,000 pounds of carbon dioxide for every megawatt-hour generated, which is almost double the approximately 1100 pounds of carbon dioxide released by a natural gas-fired electric plant per megawatt-hour generated. Because of this higher carbon efficiency of natural gas generation, as the market in the United States has changed to reduce coal and increase natural gas generation, carbon dioxide emissions have fallen. Those measured in the first quarter of 2012 were the lowest of any recorded for the first quarter of any year since 1992.[7] In 2013, the head of the UN climate agency advised that most of the world's coal reserves should be left in the ground to avoid catastrophic global warming.[8]
Coal is extracted from the ground by coal mining, either underground by shaft mining, or at ground level by open pit mining extraction. Since 1983 the world top coal producer has been China.[9] In 2011 China produced 3,520 millions of tonnes of coal – 49.5% of 7,695 millions tonnes world coal production. In 2011 other large producers were United States (993 millions tonnes), India (589), European Union (576) and Australia (416).[9] In 2010 the largest exporters were Australia with 328 million tonnes (27.1% of world coal export) and Indonesia with 316 millions tonnes (26.1%),[10] while the largest importers were Japan with 207 million tonnes (17.5% of world coal import), China with 195 million tonnes (16.6%) and South Korea with 126 million tonnes (10.7%).[11]
Formation

At various times in the geologic past, the Earth had dense forests in low-lying wetland areas. Due to natural processes such as flooding, these forests were buried underneath soil. As more and more soil deposited over them, they were compressed. The temperature also rose as they sank deeper and deeper. As the process continued the plant matter was protected from biodegradation and oxidation, usually by mud or acidic water. This trapped the carbon in immense peat bogs that were eventually covered and deeply buried by sediments. Under high pressure and high temperature, dead vegetation was slowly converted to coal. As coal contains mainly carbon, the conversion of dead vegetation into coal is called carbonization.[12]
The wide, shallow seas of the Carboniferous Period provided ideal conditions for coal formation, although coal is known from most geological periods. The exception is the coal gap in the Permian–Triassic extinction event, where coal is rare. Coal is known from Precambrian strata, which predate land plants — this coal is presumed to have originated from residues of algae.[13][14]
Types

As geological processes apply pressure to dead biotic material over time, under suitable conditions it is transformed successively into:
- Peat, considered to be a precursor of coal, has industrial importance as a fuel in some regions, for example, Ireland and Finland. In its dehydrated form, peat is a highly effective absorbent for fuel and oil spills on land and water. It is also used as a conditioner for soil to make it more able to retain and slowly release water.
- Lignite, or brown coal, is the lowest rank of coal and used almost exclusively as fuel for electric power generation. Jet, a compact form of lignite, is sometimes polished and has been used as an ornamental stone since the Upper Palaeolithic.
- Sub-bituminous coal, whose properties range from those of lignite to those of bituminous coal, is used primarily as fuel for steam-electric power generation and is an important source of light aromatic hydrocarbons for the chemical synthesis industry.
- Bituminous coal is a dense sedimentary rock, usually black, but sometimes dark brown, often with well-defined bands of bright and dull material; it is used primarily as fuel in steam-electric power generation, with substantial quantities used for heat and power applications in manufacturing and to make coke.
- "Steam coal" is a grade between bituminous coal and anthracite, once widely used as a fuel for steam locomotives. In this specialized use, it is sometimes known as "sea-coal" in the US.[15] Small steam coal (dry small steam nuts or DSSN) was used as a fuel for domestic water heating.
- Anthracite, the highest rank of coal, is a harder, glossy black coal used primarily for residential and commercial space heating. It may be divided further into metamorphically altered bituminous coal and "petrified oil", as from the deposits in Pennsylvania.
- Graphite, technically the highest rank, is difficult to ignite and is not commonly used as fuel — it is mostly used in pencils and, when powdered, as a lubricant.
The classification of coal is generally based on the content of volatiles. However, the exact classification varies between countries. According to the German classification, coal is classified as follows:[16]
| German Classification | English Designation | Volatiles % | C Carbon % | H Hydrogen % | O Oxygen % | S Sulfur % | Heat content kJ/kg |
|---|---|---|---|---|---|---|---|
| Braunkohle | Lignite (brown coal) | 45–65 | 60–75 | 6.0–5.8 | 34-17 | 0.5-3 | <28,470 |
| Flammkohle | Flame coal | 40-45 | 75-82 | 6.0-5.8 | >9.8 | ~1 | <32,870 |
| Gasflammkohle | Gas flame coal | 35-40 | 82-85 | 5.8-5.6 | 9.8-7.3 | ~1 | <33,910 |
| Gaskohle | Gas coal | 28-35 | 85-87.5 | 5.6-5.0 | 7.3-4.5 | ~1 | <34,960 |
| Fettkohle | Fat coal | 19-28 | 87.5-89.5 | 5.0-4.5 | 4.5-3.2 | ~1 | <35,380 |
| Esskohle | Forge coal | 14-19 | 89.5-90.5 | 4.5-4.0 | 3.2-2.8 | ~1 | <35,380 |
| Magerkohle | Nonbaking coal | 10-14 | 90.5-91.5 | 4.0-3.75 | 2.8-3.5 | ~1 | 35,380 |
| Anthrazit | Anthracite | 7-12 | >91.5 | <3.75 | <2.5 | ~1 | <35,300 |
| Percent by weight | |||||||
The middle six grades in the table represent a progressive transition from the English-language sub-bituminous to bituminous coal, while the last class is an approximate equivalent to anthracite, but more inclusive (US anthracite has < 6% volatiles).
Cannel coal (sometimes called "candle coal") is a variety of fine-grained, high-rank coal with significant hydrogen content. It consists primarily of "exinite" macerals, now termed "liptinite".
Hilt's law
Hilt's law is a geological term that states that, in a small area, the deeper the coal, the higher its rank (grade). The law holds true if the thermal gradient is entirely vertical, but metamorphism may cause lateral changes of rank, irrespective of depth.
Content
| Substance | Content |
|---|---|
| Mercury (Hg) | 0.10±0.01 ppm[17] |
| Arsenic (As) | 1.4 – 71 ppm[18] |
| Selenium (Se) | 3 ppm[19] |
Early uses as fuel

Coal from the Fushun mine in northeastern China was used to smelt copper as early as 1000 BCE.[20] Marco Polo, the Italian who traveled to China in the 13th century, described coal as "black stones ... which burn like logs", and said coal was so plentiful, people could take three hot baths a week.[21] In Europe, the earliest reference to the use of coal as fuel is from the geological treatise On stones (Lap. 16) by the Greek scientist Theophrastus (circa 371–287 BC):[22][23]
Among the materials that are dug because they are useful, those known as anthrakes [coals] are made of earth, and, once set on fire, they burn like charcoal. They are found in Liguria ... and in Elis as one approaches Olympia by the mountain road; and they are used by those who work in metals.
— Theophrastus, On Stones (16) translation
Outcrop coal was used in Britain during the Bronze Age (3000–2000 BC), where it has been detected as forming part of the composition of funeral pyres.[24][25] In Roman Britain, with the exception of two modern fields, "the Romans were exploiting coals in all the major coalfields in England and Wales by the end of the second century AD".[26] Evidence of trade in coal (dated to about AD 200) has been found at the Roman settlement at Heronbridge, near Chester, and in the Fenlands of East Anglia, where coal from the Midlands was transported via the Car Dyke for use in drying grain.[27] Coal cinders have been found in the hearths of villas and Roman forts, particularly in Northumberland, dated to around AD 400. In the west of England, contemporary writers described the wonder of a permanent brazier of coal on the altar of Minerva at Aquae Sulis (modern day Bath), although in fact easily accessible surface coal from what became the Somerset coalfield was in common use in quite lowly dwellings locally.[28] Evidence of coal's use for iron-working in the city during the Roman period has been found.[29] In Eschweiler, Rhineland, deposits of bituminous coal were used by the Romans for the smelting of iron ore.[26]

No evidence exists of the product being of great importance in Britain before the High Middle Ages, after about AD 1000.[30] Mineral coal came to be referred to as "seacoal" in the 13th century; the wharf where the material arrived in London was known as Seacoal Lane, so identified in a charter of King Henry III granted in 1253.[31] Initially, the name was given because much coal was found on the shore, having fallen from the exposed coal seams on cliffs above or washed out of underwater coal outcrops,[30] but by the time of Henry VIII, it was understood to derive from the way it was carried to London by sea.[32] In 1257–59, coal from Newcastle upon Tyne was shipped to London for the smiths and lime-burners building Westminster Abbey.[30] Seacoal Lane and Newcastle Lane, where coal was unloaded at wharves along the River Fleet, are still in existence.[33] (See Industrial processes below for modern uses of the term.)
These easily accessible sources had largely become exhausted (or could not meet the growing demand) by the 13th century, when underground extraction by shaft mining or adits was developed.[24] The alternative name was "pitcoal", because it came from mines. It was, however, the development of the Industrial Revolution that led to the large-scale use of coal, as the steam engine took over from the water wheel. In 1700, five-sixths of the world's coal was mined in Britain. Britain would have run out of suitable sites for watermills by the 1830s if coal had not been available as a source of energy.[34] In 1947, there were some 750,000 miners in Britain,[35] but by 2004, this had shrunk to some 5,000 miners working in around 20 collieries.[36]
Uses today


Coal as fuel
Coal is primarily used as a solid fuel to produce electricity and heat through combustion. World coal consumption was about 7.25 billion tonnes in 2010[37] (7.99 billion short tons) and is expected to increase 48% to 9.05 billion tonnes (9.98 billion short tons) by 2030.[38] China produced 3.47 billion tonnes (3.83 billion short tons) in 2011. India produced about 578 million tonnes (637.1 million short tons) in 2011. 68.7% of China's electricity comes from coal. The USA consumed about 13% of the world total in 2010, i.e. 951 million tonnes (1.05 billion short tons), using 93% of it for generation of electricity.[39] 46% of total power generated in the USA was done using coal.[40]
When coal is used for electricity generation, it is usually pulverized and then combusted (burned) in a furnace with a boiler.[41] The furnace heat converts boiler water to steam, which is then used to spin turbines which turn generators and create electricity.[42] The thermodynamic efficiency of this process has been improved over time; some older coal-fired power stations have thermal efficiencies in the vicinity of 25%[43] whereas the newest supercritical and "ultra-supercritical" steam cycle turbines, operating at temperatures over 600 °C and pressures over 27 MPa (over 3900 psi), can practically achieve thermal efficiencies in excess of 45% (LHV basis) using anthracite fuel,[44][45] or around 43% (LHV basis) even when using lower-grade lignite fuel.[46] Further thermal efficiency improvements are also achievable by improved pre-drying (especially relevant with high-moisture fuel such as lignite or biomass) and cooling technologies.[47]
An alternative approach of using coal for electricity generation with improved efficiency is the integrated gasification combined cycle (IGCC) power plant. Instead of pulverizing the coal and burning it directly as fuel in the steam-generating boiler, the coal can be first gasified (see coal gasification) to create syngas, which is burned in a gas turbine to produce electricity (just like natural gas is burned in a turbine). Hot exhaust gases from the turbine are used to raise steam in a heat recovery steam generator which powers a supplemental steam turbine. Thermal efficiencies of current IGCC power plants range from 39-42%[48] (HHV basis) or ~42-45% (LHV basis) for bituminous coal and assuming utilization of mainstream gasification technologies (Shell, GE Gasifier, CB&I). IGCC power plants outperform conventional pulverized coal-fueled plants in terms of pollutant emissions, and allow for relatively easy carbon capture.
At least 40% of the world's electricity comes from coal,[41][49] and in 2012, about one-third of the United States' electricity came from coal, down from approximately 49% in 2008.[50][51] As of 2012 in the United States, use of coal to generate electricity was declining, as plentiful supplies of natural gas obtained by hydraulic fracturing of tight shale formations became available at low prices.[50]
In Denmark, a net electric efficiency of > 47% has been obtained at the coal-fired Nordjyllandsværket CHP Plant and an overall plant efficiency of up to 91% with cogeneration of electricity and district heating.[52] The multifuel-fired Avedøreværket CHP Plant just outside Copenhagen can achieve a net electric efficiency as high as 49%. The overall plant efficiency with cogeneration of electricity and district heating can reach as much as 94%.[53]
An alternative form of coal combustion is as coal-water slurry fuel (CWS), which was developed in the Soviet Union. CWS significantly reduces emissions, improving the heating value of coal.[citation needed] Other ways to use coal are combined heat and power cogeneration and an MHD topping cycle.
The total known deposits recoverable by current technologies, including highly polluting, low-energy content types of coal (i.e., lignite, bituminous), is sufficient for many years.[quantify] However, consumption is increasing and maximal production could be reached within decades (see world coal reserves, below). On the other hand much may have to be left in the ground to avoid climate change.[54][55]
Coking coal and use of coke

Coke is a solid carbonaceous residue derived from low-ash, low-sulfur bituminous coal from which the volatile constituents are driven off by baking in an oven without oxygen at temperatures as high as 1,000 °C (1,832 °F), so the fixed carbon and residual ash are fused together. Metallurgical coke is used as a fuel and as a reducing agent in smelting iron ore in a blast furnace.[56] The result is pig iron, and is too rich in dissolved carbon, so it must be treated further to make steel. The coking coal should be low in sulfur and phosphorus, so they do not migrate to the metal.
The coke must be strong enough to resist the weight of overburden in the blast furnace, which is why coking coal is so important in making steel using the conventional route. However, the alternative route is direct reduced iron, where any carbonaceous fuel can be used to make sponge or pelletised iron. Coke from coal is grey, hard, and porous and has a heating value of 24.8 million Btu/ton (29.6 MJ/kg). Some cokemaking processes produce valuable byproducts, including coal tar, ammonia, light oils, and coal gas.
Petroleum coke is the solid residue obtained in oil refining, which resembles coke, but contains too many impurities to be useful in metallurgical applications.
Gasification
Coal gasification can be used to produce syngas, a mixture of carbon monoxide (CO) and hydrogen (H2) gas. Often syngas is used to fire gas turbines to produce electricity, but the versatility of syngas also allows it to be converted into transportation fuels, such as gasoline and diesel, through the Fischer-Tropsch process; alternatively, syngas can be converted into methanol, which can be blended into fuel directly or converted to gasoline via the methanol to gasoline process.[57] Gasification combined with Fischer-Tropsch technology is currently used by the Sasol chemical company of South Africa to make motor vehicle fuels from coal and natural gas. Alternatively, the hydrogen obtained from gasification can be used for various purposes, such as powering a hydrogen economy, making ammonia, or upgrading fossil fuels.
During gasification, the coal is mixed with oxygen and steam while also being heated and pressurized. During the reaction, oxygen and water molecules oxidize the coal into carbon monoxide (CO), while also releasing hydrogen gas (H2). This process has been conducted in both underground coal mines and in the production of town gas.
- C (as Coal) + O2 + H2O → H2 + CO
If the refiner wants to produce gasoline, the syngas is collected at this state and routed into a Fischer-Tropsch reaction. If hydrogen is the desired end-product, however, the syngas is fed into the water gas shift reaction, where more hydrogen is liberated.
- CO + H2O → CO2 + H2
In the past, coal was converted to make coal gas (town gas), which was piped to customers to burn for illumination, heating, and cooking.
Liquefaction
Coal can also be converted into synthetic fuels equivalent to gasoline or diesel by several different direct processes (which do not intrinsically require gasification or indirect conversion).[58] In the direct liquefaction processes, the coal is either hydrogenated or carbonized. Hydrogenation processes are the Bergius process,[59] the SRC-I and SRC-II (Solvent Refined Coal) processes, the NUS Corporation hydrogenation process[60][61] and several other single-stage and two-stage processes.[62] In the process of low-temperature carbonization, coal is coked at temperatures between 360 and 750 °C (680 and 1,380 °F). These temperatures optimize the production of coal tars richer in lighter hydrocarbons than normal coal tar. The coal tar is then further processed into fuels. An overview of coal liquefaction and its future potential is available.[63]
Coal liquefaction methods involve carbon dioxide (CO2) emissions in the conversion process. If coal liquefaction is done without employing either carbon capture and storage (CCS) technologies or biomass blending, the result is lifecycle greenhouse gas footprints that are generally greater than those released in the extraction and refinement of liquid fuel production from crude oil. If CCS technologies are employed, reductions of 5–12% can be achieved in Coal to Liquid (CTL) plants and up to a 75% reduction is achievable when co-gasifying coal with commercially demonstrated levels of biomass (30% biomass by weight) in coal/biomass-to-liquids plants.[64] For future synthetic fuel projects, carbon dioxide sequestration is proposed to avoid releasing CO2 into the atmosphere. Sequestration adds to the cost of production.
Refined coal
Refined coal is the product of a coal-upgrading technology that removes moisture and certain pollutants from lower-rank coals such as sub-bituminous and lignite (brown) coals. It is one form of several precombustion treatments and processes for coal that alter coal's characteristics before it is burned. The goals of precombustion coal technologies are to increase efficiency and reduce emissions when the coal is burned. Depending on the situation, precombustion technology can be used in place of or as a supplement to postcombustion technologies to control emissions from coal-fueled boilers.
Industrial processes
Finely ground bituminous coal, known in this application as sea coal, is a constituent of foundry sand. While the molten metal is in the mould, the coal burns slowly, releasing reducing gases at pressure, and so preventing the metal from penetrating the pores of the sand. It is also contained in 'mould wash', a paste or liquid with the same function applied to the mould before casting.[65] Sea coal can be mixed with the clay lining (the "bod") used for the bottom of a cupola furnace. When heated, the coal decomposes and the bod becomes slightly friable, easing the process of breaking open holes for tapping the molten metal.[66]
Production of chemicals[67]
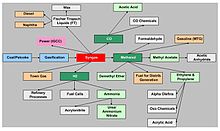
Coal is an important feedstock in production of a wide range of chemical fertilizers and other chemical products. The main route to these products is coal gasification to produce syngas. Primary chemicals that are produced directly from the syngas include methanol, hydrogen and carbon monoxide, which are the chemical building blocks from which a whole spectrum of derivative chemicals are manufactured, including olefins, acetic acid, formaldehyde, ammonia, urea and others. The versatility of syngas as a precursor to primary chemicals and high-value derivative products provides the option of using relatively inexpensive coal to produce a wide range of valuable commodities.
Historically, production of chemicals from coal has been used since the 1950s and has become established in the market. According to the 2010 Worldwide Gasification Database,[68] a survey of current and planned gasifiers, from 2004 to 2007 chemical production increased its gasification product share from 37% to 45%. From 2008 to 2010, 22% of new gasifier additions were to be for chemical production.
Because the slate of chemical products that can be made via coal gasification can in general also use feedstocks derived from natural gas and petroleum, the chemical industry tends to use whatever feedstocks are most cost-effective. Therefore, interest in using coal tends to increase for higher oil and natural gas prices and during periods of high global economic growth that may strain oil and gas production. Also, production of chemicals from coal is of much higher interest in countries like South Africa, China, India and the United States where there are abundant coal resources. The abundance of coal combined with lack of natural gas resources in China is strong inducement for the coal to chemicals industry pursued there. In the United States, the best example of the industry is Eastman Chemical Company which has been successfully operating a coal-to-chemicals plant at its Kingsport, Tennessee, site since 1983. Similarly, Sasol has built and operated coal-to-chemicals facilities in South Africa.
Coal to chemical processes do require substantial quantities of water. As of 2013 much of the coal to chemical production was in the People's Republic of China[69][70] where environmental regulation and water management[71] was weak.[72]
Cultural usage
Coal is the official state mineral of Kentucky.[73] and the official state rock of Utah;[74] both U.S. states have a historic link to coal mining.
Some cultures hold that children who misbehave will receive only a lump of coal from Santa Claus for Christmas in their christmas stockings instead of presents.
It is also customary and considered lucky in Scotland and the North of England to give coal as a gift on New Year's Day. This occurs as part of First-Footing and represents warmth for the year to come.
Coal as a traded commodity
In North America, Central Appalachian coal futures contracts are currently traded on the New York Mercantile Exchange (trading symbol QL). The trading unit is 1,550 short tons (1,410 t) per contract, and is quoted in U.S. dollars and cents per ton. Since coal is the principal fuel for generating electricity in the United States, coal futures contracts provide coal producers and the electric power industry an important tool for hedging and risk management.[75]
In addition to the NYMEX contract, the IntercontinentalExchange (ICE) has European (Rotterdam) and South African (Richards Bay) coal futures available for trading. The trading unit for these contracts is 5,000 tonnes (5,500 short tons), and are also quoted in U.S. dollars and cents per ton.[76]
The price of coal increased from around $30.00 per short ton in 2000 to around $150.00 per short ton as of September 2008. As of October 2008, the price per short ton had declined to $111.50. Prices further declined to $71.25 as of October 2010.[77]
Environmental effects

A number of adverse health,[78] and environmental effects of coal burning exist,[79] especially in power stations, and of coal mining, including:
- Coal-fired power plants cause nearly 24,000 premature deaths annually in the United States, including 2,800 from lung cancer.[80] Annual health costs in Europe from use of coal to generate electricity are €42.8 billion, or $55 billion.[81]
- Generation of hundreds of millions of tons of waste products, including fly ash, bottom ash, and flue-gas desulfurization sludge, that contain mercury, uranium, thorium, arsenic, and other heavy metals
- Acid rain from high sulfur coal
- Interference with groundwater and water table levels due to mining
- Contamination of land and waterways and destruction of homes from fly ash spills. such as the Kingston Fossil Plant coal fly ash slurry spill
- Impact of water use on flows of rivers and consequential impact on other land uses
- Dust nuisance
- Subsidence above tunnels, sometimes damaging infrastructure
- Uncontrollable coal seam fire which may burn for decades or centuries
- Coal-fired power plants without effective fly ash capture systems are one of the largest sources of human-caused background radiation exposure.
- Coal-fired power plants emit mercury, selenium, and arsenic, which are harmful to human health and the environment.[82]
- Release of carbon dioxide, a greenhouse gas, causes climate change and global warming, according to the IPCC and the EPA. Coal is the largest contributor to the human-made increase of CO2 in the atmosphere.[83]
- Approximately 75 Tg/S per year of sulfur dioxide (SO2) is released from burning coal. After release, the sulfur dioxide is oxidized to gaseous H2SO2 which scatters solar radiation, hence its increase in the atmosphere exerts a cooling effect on climate that masks some of the warming caused by increased greenhouse gases. Release of SO2 also contributes to the widespread acidification of ecosystems.[84]
Bioremediation
The white rot fungus C. versicolor can grow on and metabolize naturally occcuring coal.[85] The bacteria Diplococcus has been found to degrade coal, raising its temperature.[86]
Economic aspects
Coal (by liquefaction technology) is one of the backstop resources that could limit escalation of oil prices and mitigate the effects of transportation energy shortage that will occur under peak oil. This is contingent on liquefaction production capacity becoming large enough to satiate the very large and growing demand for petroleum. Estimates of the cost of producing liquid fuels from coal suggest that domestic U.S. production of fuel from coal becomes cost-competitive with oil priced at around $35 per barrel,[87] with the $35 being the break-even cost. With oil prices as low as around $40 per barrel in the U.S. as of December 2008, liquid coal lost some of its economic allure in the U.S., but will probably be re-vitalized, similar to oil sand projects, with an oil price around $70 per barrel.
In China, due to an increasing need for liquid energy in the transportation sector, coal liquefaction projects were given high priority even during periods of oil prices below $40 per barrel.[88] This is probably because China prefers not to be dependent on foreign oil, instead utilizing its enormous domestic coal reserves. As oil prices were increasing during the first half of 2009, the coal liquefaction projects in China were again boosted, and these projects are profitable with an oil barrel price of $40.[89]
China is the largest producer of coal in the world. It is the world's largest energy consumer, and relies on coal to supply 69% of its energy needs.[90] An estimated 5 million people worked in China's coal-mining industry in 2007.[91]
Coal pollution costs the EU €43 billion each year.[92] Measures to cut air pollution may have beneficial long-term economic impacts for individuals.[93]
Energy density and carbon impact
The energy density of coal, i.e. its heating value, is roughly 24 megajoules per kilogram[94] (approximately 6.7 kilowatt-hours per kg). For a coal power plant with a 40% efficiency, it takes an estimated 325 kg (717 lb) of coal to power a 100 W lightbulb for one year.[95]
As of 2006, the average efficiency of electricity-generating power stations was 31%; in 2002, coal represented about 23% of total global energy supply, an equivalent of 3.4 billion tonnes of coal, of which 2.8 billion tonnes were used for electricity generation.[96]
The US Energy Information Agency's 1999 report on CO2 emissions for energy generation quotes an emission factor of 0.963 kg CO2/kWh for coal power, compared to 0.881 kg CO2/kWh (oil), or 0.569 kg CO2/kWh (natural gas).[97]
Underground fires
Thousands of coal fires are burning around the world.[98] Those burning underground can be difficult to locate and many cannot be extinguished. Fires can cause the ground above to subside, their combustion gases are dangerous to life, and breaking out to the surface can initiate surface wildfires. Coal seams can be set on fire by spontaneous combustion or contact with a mine fire or surface fire. Lightning strikes are an important source of ignition. The coal continues to burn slowly back into the seam until oxygen (air) can no longer reach the flame front. A grass fire in a coal area can set dozens of coal seams on fire.[99][100] Coal fires in China burn an estimated 120 million tons of coal a year, emitting 360 million metric tons of CO2, amounting to 2–3% of the annual worldwide production of CO2 from fossil fuels.[101][102] In Centralia, Pennsylvania (a borough located in the Coal Region of the United States), an exposed vein of anthracite ignited in 1962 due to a trash fire in the borough landfill, located in an abandoned anthracite strip mine pit. Attempts to extinguish the fire were unsuccessful, and it continues to burn underground to this day. The Australian Burning Mountain was originally believed to be a volcano, but the smoke and ash comes from a coal fire that has been burning for some 6,000 years.[103]
At Kuh i Malik in Yagnob Valley, Tajikistan, coal deposits have been burning for thousands of years, creating vast underground labyrinths full of unique minerals, some of them very beautiful. Local people once used this method to mine ammoniac. This place has been well-known since the time of Herodotus, but European geographers misinterpreted the Ancient Greek descriptions as the evidence of active volcanism in Turkestan (up to the 19th century, when the Russian army invaded the area).[citation needed]
The reddish siltstone rock that caps many ridges and buttes in the Powder River Basin in Wyoming and in western North Dakota is called porcelanite, which resembles the coal burning waste "clinker" or volcanic "scoria".[104] Clinker is rock that has been fused by the natural burning of coal. In the Powder River Basin approximately 27 to 54 billion tons of coal burned within the past three million years.[105] Wild coal fires in the area were reported by the Lewis and Clark Expedition as well as explorers and settlers in the area.[106]
Production trends



In 2006, China was the top producer of coal with 38% share followed by the United States and India, according to the British Geological Survey. As of 2012 coal production in the United States was falling at the rate of 7% annually[107] with many power plants using coal shut down or converted to natural gas; however, some of the reduced domestic demand was taken up by increased exports[108] with five coal export terminals being proposed in the Pacific Northwest to export coal from the Powder River Basin to China and other Asian markets;[109] however, as of 2013, environmental opposition was increasing.[110] High-sulfur coal mined in Illinois which was unsaleable in the United States found a ready market in Asia as exports reached 13 million tons in 2012.[111]
World coal reserves
The 948 billion short tons of recoverable coal reserves estimated by the Energy Information Administration are equal to about 4,196 BBOE (billion barrels of oil equivalent).[3] The amount of coal burned during 2007 was estimated at 7.075 billion short tons, or 133.179 quadrillion BTU's.[112] This is an average of 18.8 million BTU per short ton. In terms of heat content, this is about 57,000,000 barrels (9,100,000 m3) of oil equivalent per day. By comparison in 2007, natural gas provided 51,000,000 barrels (8,100,000 m3) of oil equivalent per day, while oil provided 85,800,000 barrels (13,640,000 m3) per day.
British Petroleum, in its 2007 report, estimated at 2006 end that there were 147 years reserves-to-production ratio based on proven coal reserves worldwide. This figure only includes reserves classified as "proven"; exploration drilling programs by mining companies, particularly in under-explored areas, are continually providing new reserves. In many cases, companies are aware of coal deposits that have not been sufficiently drilled to qualify as "proven". However, some nations haven't updated their information and assume reserves remain at the same levels even with withdrawals. Energy Watch Group predicted in 2007 that peak coal production may occur sometime around 2025, depending on future coal production rates.[113]
Of the three fossil fuels, coal has the most widely distributed reserves; coal is mined in over 100 countries, and on all continents except Antarctica. The largest reserves are found in the United States, Russia, China, Australia and India. Note the table below.
| Country | Anthracite & Bituminous | SubBituminous | Lignite | Total | Percentage of World Total |
|---|---|---|---|---|---|
| 108,501 | 98,618 | 30,176 | 237,295 | 22.6 | |
| 49,088 | 97,472 | 10,450 | 157,010 | 14.4 | |
| 62,200 | 33,700 | 18,600 | 114,500 | 12.6 | |
| 37,100 | 2,100 | 37,200 | 76,400 | 8.9 | |
| 56,100 | 0 | 4,500 | 60,600 | 7.0 | |
| 99 | 0 | 40,600 | 40,699 | 4.7 | |
| 15,351 | 16,577 | 1,945 | 33,873 | 3.9 | |
| 21,500 | 0 | 12,100 | 33,600 | 3.9 | |
| 30,156 | 0 | 0 | 30,156 | 3.5 | |
| 9 | 361 | 13,400 | 13,770 | 1.6 | |
| 6,366 | 380 | 0 | 6,746 | 0.8 | |
| 3,474 | 872 | 2,236 | 6,528 | 0.8 | |
| 4,338 | 0 | 1,371 | 5,709 | 0.7 | |
| 1,520 | 2,904 | 1,105 | 5,529 | 0.6 | |
| 0 | 4,559 | 0 | 4,559 | 0.5 | |
| 0 | 0 | 3,020 | 3,020 | 0.4 | |
| 484 | 0 | 2,369 | 2,853 | 0.3 | |
| 1,170 | 0 | 1,350 | 2,520 | 0.3 | |
| 2 | 190 | 2,174 | 2,366 | 0.3 | |
| 0 | 166 | 1,904 | 2,070 | 0.3 | |
| 529 | 0 | 1,814 | 2,343 | 0.3 | |
| 47 | 0 | 1,853 | 1,900 | 0.2 | |
| 13 | 439 | 1,208 | 1,660 | 0.2 | |
| 0 | 0 | 1,239 | 1,239 | 0.1 | |
| 860 | 300 | 51 | 1,211 | 0.1 | |
| 1,203 | 0 | 0 | 1,203 | 0.1 | |
| 192 | 0 | 908 | 1,100 | 0.1 | |
| 0 | 0 | 812 | 812 | 0.1 | |
| 0 | 0 | 794 | 794 | 0.1 | |
| 300 | 300 | 0 | 600 | 0.1 | |
| 33 | 205 | 333-7,000 | 571–15,000[115] | 0.1 | |
| 200 | 300 | 30 | 530 | 0.1 | |
| 4 | 0 | 499 | 503 | 0.1 | |
| 502 | 0 | 0 | 502 | 0.1 | |
| 0 | 0 | 500 | 500 | 0.1 | |
| All others | 3,421 | 1,346 | 846 | 5,613 | 0.7 |
| World Total | 404,762 | 260,789 | 195,387 | 860,938 | 100 |
Major coal producers
The reserve life is an estimate based only on current production levels and proved reserves level for the countries shown, and makes no assumptions of future production or even current production trends. Countries with annual production higher than 100 million tonnes are shown. For comparison, data for the European Union is also shown. Shares are based on data expressed in tonnes oil equivalent.
| Country | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | Share | Reserve Life (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1834.9 | 2122.6 | 2349.5 | 2528.6 | 2691.6 | 2802.0 | 2973.0 | 3235.0 | 3520.0 | 49.5% | 35 | |
| 972.3 | 1008.9 | 1026.5 | 1054.8 | 1040.2 | 1063.0 | 975.2 | 983.7 | 992.8 | 14.1% | 239 | |
| 375.4 | 407.7 | 428.4 | 449.2 | 478.4 | 515.9 | 556.0 | 573.8 | 588.5 | 5.6% | 103 | |
| 637.2 | 627.6 | 607.4 | 595.1 | 592.3 | 563.6 | 538.4 | 535.7 | 576.1 | 4.2% | 97 | |
| 350.4 | 364.3 | 375.4 | 382.2 | 392.7 | 399.2 | 413.2 | 424.0 | 415.5 | 5.8% | 184 | |
| 276.7 | 281.7 | 298.3 | 309.9 | 313.5 | 328.6 | 301.3 | 321.6 | 333.5 | 4.0% | 471 | |
| 114.3 | 132.4 | 152.7 | 193.8 | 216.9 | 240.2 | 256.2 | 275.2 | 324.9 | 5.1% | 17 | |
| 237.9 | 243.4 | 244.4 | 244.8 | 247.7 | 252.6 | 250.6 | 254.3 | 255.1 | 3.6% | 118 | |
| 204.9 | 207.8 | 202.8 | 197.1 | 201.9 | 192.4 | 183.7 | 182.3 | 188.6 | 1.1% | 216 | |
| 163.8 | 162.4 | 159.5 | 156.1 | 145.9 | 144.0 | 135.2 | 133.2 | 139.2 | 1.4% | 41 | |
| 84.9 | 86.9 | 86.6 | 96.2 | 97.8 | 111.1 | 100.9 | 110.9 | 115.9 | 1.5% | 290 | |
| World Total | 5,301.3 | 5,716.0 | 6,035.3 | 6,342.0 | 6,573.3 | 6,795.0 | 6,880.8 | 7,254.6 | 7,695.4 | 100% | 112 |
Major coal consumers
Countries with annual consumption higher than 20 million tonnes are shown.
| Country | 2008 | 2009 | 2010 | 2011 | Share |
|---|---|---|---|---|---|
| 2,966 | 3,188 | 3,695 | 4,053 | 50.7% | |
| 1,121 | 997 | 1,048 | 1,003 | 12.5% | |
| 641 | 705 | 722 | 788 | 9.9% | |
| 250 | 204 | 256 | 262 | 3.3% | |
| 268 | 248 | 256 | 256 | 3.3% | |
| 215 | 204 | 206 | 210 | 2.6% | |
| 204 | 181 | 206 | 202 | 2.5% | |
| 149 | 151 | 149 | 162 | 2.0% | |
| World Total | 7,327 | 7,318 | 7,994 | N/A | 100% |
Major coal exporters
Countries with annual gross export higher than 10 million tonnes are shown. In terms of net export the largest exporters are still Australia (328.1 millions tonnes), Indonesia (316.2) and Russia (100.2).
| Country | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Share |
|---|---|---|---|---|---|---|---|---|---|
| 238.1 | 247.6 | 255.0 | 255.0 | 268.5 | 278.0 | 288.5 | 328.1 | 27.1% | |
| 107.8 | 131.4 | 142.0 | 192.2 | 221.9 | 228.2 | 261.4 | 316.2 | 26.1% | |
| 41.0 | 55.7 | 98.6 | 103.4 | 112.2 | 115.4 | 130.9 | 122.1 | 10.1% | |
| 43.0 | 48.0 | 51.7 | 51.2 | 60.6 | 83.5 | 60.4 | 83.2 | 6.9% | |
| 78.7 | 74.9 | 78.8 | 75.8 | 72.6 | 68.2 | 73.8 | 76.7 | 6.3% | |
| 50.4 | 56.4 | 59.2 | 68.3 | 74.5 | 74.7 | 75.7 | 76.4 | 6.3% | |
| 27.7 | 28.8 | 31.2 | 31.2 | 33.4 | 36.5 | 31.9 | 36.9 | 3.0% | |
| 30.3 | 27.4 | 28.3 | 30.5 | 32.8 | 47.6 | 33.0 | 36.3 | 3.0% | |
| 6.9 | 11.7 | 19.8 | 23.5 | 35.1 | 21.3 | 28.2 | 24.7 | 2.0% | |
| 103.4 | 95.5 | 93.1 | 85.6 | 75.4 | 68.8 | 25.2 | 22.7 | 1.9% | |
| 0.5 | 1.7 | 2.3 | 2.5 | 3.4 | 4.4 | 7.7 | 18.3 | 1.5% | |
| 28.0 | 27.5 | 26.5 | 25.4 | 20.1 | 16.1 | 14.6 | 18.1 | 1.5% | |
| Total | 713.9 | 764.0 | 936.0 | 1,000.6 | 1,073.4 | 1,087.3 | 1,090.8 | 1,212.8 | 100% |
Major coal importers
Countries with annual gross import higher than 20 million tonnes are shown. In terms of net import the largest importers are still Japan (206.0 millions tonnes), China (172.4) and South Korea (125.8).[119]
| Country | 2006 | 2007 | 2008 | 2009 | 2010 | Share |
|---|---|---|---|---|---|---|
| 199.7 | 209.0 | 206.0 | 182.1 | 206.7 | 17.5% | |
| 42.0 | 56.2 | 44.5 | 151.9 | 195.1 | 16.6% | |
| 84.1 | 94.1 | 107.1 | 109.9 | 125.8 | 10.7% | |
| 52.7 | 29.6 | 70.9 | 76.7 | 101.6 | 8.6% | |
| 69.1 | 72.5 | 70.9 | 64.6 | 71.1 | 6.0% | |
| 50.6 | 56.2 | 55.7 | 45.9 | 55.1 | 4.7% | |
| 22.9 | 25.8 | 21.7 | 22.7 | 30.0 | 2.5% | |
| 56.8 | 48.9 | 49.2 | 42.2 | 29.3 | 2.5% | |
| 27.9 | 28.0 | 27.9 | 20.9 | 23.7 | 1.9% | |
| 25.7 | 29.3 | 23.5 | 22.1 | 22.8 | 1.9% | |
| 28.8 | 26.3 | 34.6 | 26.8 | 21.8 | 1.9% | |
| 24.1 | 22.1 | 24.9 | 18.3 | 20.8 | 1.8% | |
| 40.3 | 38.8 | 37.8 | 23.1 | 20.6 | 1.8% | |
| Total | 991.8 | 1,056.5 | 1,063.2 | 1,039.8 | 1,178.1 | 100% |
See also
- Asphaltene
- Biochar
- Biomass-coal
- Carbochemistry
- Clean coal
- Coal assay
- Coal dust
- Coal homogenization
- Coal Measure (stratigraphic unit)
- Coal mining
- Coal phase out
- Coal-tar
- Coalbed methane
- Fluidized bed combustion
- Gytta
- Major coal producing regions
- Mountaintop removal mining
- Tonstein
- The Coal Question
- World Coal Association
- Fossil fuels
- Oil (petroleum)
References
- ^ Harper, Douglas. "coal". Online Etymology Dictionary.
- ^ Blander, M. "Calculations of the Influence of Additives on Coal Combustion Deposits" (PDF). Argonne National Laboratory. p. 315. Retrieved 17 December 2011.
- ^ a b "EIA International Energy Statistics : Coal : Recoverable Reserves". Retrieved 31 May 2012.
- ^ Fred Pearce (15 February 2014). "Fire in the hole: After fracking comes coal". New Scientist: 36–41.
- ^ "International Energy Annual 2006". Energy Information Administration. 2008. Archived from the original on 23 May 2011.
{{cite journal}}: Cite journal requires|journal=(help), see data tables - ^ "International energy statistics". U.S. Energy Information Administration. Retrieved 10 March 2014., see table
- ^ Nuwer, Rachel (2012-08-17). A 20-Year Low in U.S. Carbon Emissions. blogs.nytimes.com
- ^ "Leave coal in the ground to avoid climate catastrophe, UN tells industry".
- ^ a b c "BP Statistical review of world energy 2012" (XLS). British Petroleum. Retrieved 18 August 2011.
- ^ a b EIA International Energy Annual – Total Coal Exports (Thousand Short Tons). Tonto.eia.doe.gov. Retrieved on 24 August 2012.
- ^ a b International Energy Annual – Total Coal Imports (Thousand Short Tons). Tonto.eia.doe.gov. Retrieved on 24 August 2012.
- ^ Taylor, Thomas N; Taylor, Edith L; Krings, Michael (2009). Paleobotany: The biology and evolution of fossil plants. ISBN 978-0-12-373972-8.
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1130/0016-7606(1957)68[1293:ACFPUH]2.0.CO;2, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1130/0016-7606(1957)68[1293:ACFPUH]2.0.CO;2instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.2113/gsecongeo.76.4.951, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.2113/gsecongeo.76.4.951instead. - ^ Funk and Wagnalls, quoted in "sea-coal". Oxford English Dictionary (2 ed.). Oxford University Press. 1989.
- ^ Eberhard Lindner; Chemie für Ingenieure; Lindner Verlag Karlsruhe, S. 258
- ^ "Mercury in coal: a review ; Part 1. Geochemistry ; Ya. E. Yudovich*, M.P. Ketris" (PDF). labtechgroup.com. 21 April 2010. Retrieved 22 February 2013.
- ^ "Arsenic in Coal" (PDF). pubs.usgs.gov. 28 March 2006. Retrieved 22 February 2013.
- ^ "Selenium in Our Environment – Trace Elements in the Environment – Advances in Chemistry (ACS Publications)". pubs.acs.org. 73-06-01. doi:10.1021/ba-1973-0123.ch006.
{{cite web}}:|access-date=requires|url=(help); Check date values in:|date=(help); Missing or empty|url=(help) - ^ coal. Encyclopedia Britannica.
- ^ Marco Polo In China. Facts and Details. Retrieved on 2013-05-11.
- ^ Carol, Mattusch (2008). Oleson, John Peter (ed.). Metalworking and Tools. Oxford University Press. pp. 418–38 (432). ISBN 978-0-19-518731-1Template:Inconsistent citations
{{cite book}}:|work=ignored (help)CS1 maint: postscript (link) - ^ Irby-Massie, Georgia L.; Keyser, Paul T. (2002). Greek Science of the Hellenistic Era: A Sourcebook. Routledge. 9.1 "Theophrastos", p.228. ISBN 0-415-23847-1Template:Inconsistent citations
{{cite book}}: CS1 maint: postscript (link) - ^ a b Britannica 2004: Coal mining: ancient use of outcropping coal
- ^ Needham, Joseph; Golas, Peter J (1999). Science and Civilisation in China. Cambridge University Press. pp. 186–91. ISBN 978-0-521-58000-7.
- ^ a b Smith, A. H. V. (1997). "Provenance of Coals from Roman Sites in England and Wales". Britannia. 28: 297–324 (322–4). doi:10.2307/526770. JSTOR 526770.
- ^ Salway, Peter (2001). A History of Roman Britain. Oxford University Press. ISBN 0-19-280138-4.
- ^ Forbes, RJ (1966): Studies in Ancient Technology. Brill Academic Publishers, Boston.
- ^ Cunliffe, Barry W. (1984). Roman Bath Discovered. London: Routledge. pp. 14–5, 194. ISBN 0-7102-0196-6.
- ^ a b c Cantril, T. C. (1914). Coal Mining. Cambridge, England: Cambridge University Press. pp. 3–10. OCLC 156716838.
- ^ "coal, 5a". Oxford English Dictionary. Oxford University Press. 1 December 2010.
- ^ John Caius, quoted in Cantril (1914).
- ^ Trench, Richard; Hillman, Ellis (1993). London under London: a subterranean guide (Second ed.). London: John Murray. p. 33. ISBN 0-7195-5288-5.
- ^ Wrigley, EA (1990). Continuity, Chance and Change: The Character of the Industrial Revolution in England. Cambridge University Press. ISBN 0-521-39657-3.
- ^ "The fall of King Coal". BBC News. 6 December 1999.
- ^ "The miners' strike revisited". Inside Out. BBC. 2 February 2004.
- ^ World coal consumption 2000–2011 EIA statistics
- ^ EIA, World Energy Projections Plus (2009)
- ^ U.S. Coal Supply and Demand: 2010 Year in Review. Eia.gov (2011-06-01). Retrieved on 24 August 2012.
- ^ Share of electric power sector net generation by energy source. Eia.gov (2011-06-01). Retrieved on 24 August 2012.
- ^ a b Total World Electricity Generation by Fuel (2006). Source: IEA 2008.
- ^ "Fossil Power Generation". Siemens AG. Retrieved 23 April 2009.
- ^ J. Nunn, A. Cottrell, A. Urfer, L. Wibberley and P. Scaife, "A Lifecycle Assessment of the Victorian Energy Grid", Cooperative Research Centre for Coal in Sustainable Development, February 2003, page 7.
- ^ Jens Rosenkranz; Andreas Wichtmann. "Balancing economics and environmental friendliness – the challenge for supercritical coal-fired power plants with highest steam parameters in the future" (PDF). Retrieved 23 October 2006.
- ^ "Lünen – State-of-theArt Ultra Supercritical Steam Power Plant Under Construction" (PDF). Siemens AG. Retrieved 21 July 2014.
- ^ "Neurath F and G set new benchmarks" (PDF). Alstom. Retrieved 21 July 2014.
- ^ "The Niederraussem Coal Innovation Centre" (PDF). RWE. Retrieved 21 July 2014.
- ^ "IGCC Efficiency/Performance". National Energy Technology Laboratory. Retrieved 16 July 2014.
- ^ coal reserves, coal exploration – World Coal Association. Worldcoal.org. Retrieved on 24 August 2012.
- ^ a b Lipton, Eric (29 May 2012). "Even in Coal Country, the Fight for an Industry". The New York Times. Retrieved 30 May 2012.
- ^ "Figure ES 1. U.S. Electric Power Industry Net Generation". Electric Power Annual with data for 2008. U.S. Energy Information Administration. 21 January 2010. Retrieved 7 November 2010.
- ^ Nordjyllandsværket. (PDF) . Retrieved on 2013-05-11.
- ^ Avedøreværket. Ipaper.ipapercms.dk. Retrieved on 2013-05-11.
- ^ http://www.newscientist.com/article/mg22029470.300-mining-insider-leave-the-coal-in-the-ground.html
- ^ "IPCC digested: Just leave the fossil fuels underground". New Scientist.
- ^ Blast furnace steelmaking cost model. Steelonthenet.com. Retrieved on 24 August 2012.
- ^ "Conversion of Methanol to Gasoline". National Energy Technology Laboratory. Retrieved 16 July 2014.
- ^ "Direct Liquefaction Processes". National Energy Technology Laboratory. Retrieved 16 July 2014.
- ^ Haul, Robert (April 1985). "Friedrich Bergius (1884–1949)". Chemie in unserer Zeit. 19. VCH-Verlagsgesellschaft mbH: 62. doi:10.1002/ciuz.19850190205.
- ^ Speight, James G. (2008). Synthetic Fuels Handbook: Properties, Process, and Performance. McGraw-Hill Professional. pp. 9–10. ISBN 978-0-07-149023-8.
- ^
Lowe, Phillip A.; Schroeder, Wilburn C.; Liccardi, Anthony L. (1976). "Technical Economies, Synfuels and Coal Energy Symposium, Solid-Phase Catalytic Coal Liquefaction Process". American Society of Mechanical Engineers: 35.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ^ "Direct Liquefaction Processes, Single-stage Processes and Two-stage Processes". National Energy Technology Laboratory. Retrieved 16 July 2014.
- ^ Höök, M., Aleklett, K. (2010). "A review on coal-to-liquid fuels and its coal consumption" (PDF). International Journal of Energy Research. 34 (10): 848. doi:10.1002/er.1596.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
Tarka, Thomas J.; Wimer, John G.; Balash, Peter C.; Skone, Timothy J.; Kern, Kenneth C.; Vargas, Maria C.; Morreale, Bryan D.; White III, Charles W.; Gray, David (2009). "Affordable Low Carbon Diesel from Domestic Coal and Biomass". United States Department of Energy, National Energy Technology Laboratory: 21.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ^ Rao, P. N. (2007). "Moulding materials". Manufacturing technology: foundry, forming and welding (2 ed.). New Delhi: Tata McGraw-Hill. p. 107. ISBN 978-0-07-463180-5.
- ^ Kirk, Edward (1899). "Cupola management". Cupola Furnace – A Practical Treatise on the Construction and Management of Foundry Cupolas. Philadelphia, PA: Baird. p. 95. OCLC 2884198.
- ^ "Chemicals". National Energy Technology Laboratory. Retrieved 12 July 2014.
- ^ "Gasification Systems 2010 Worldwide Gasification Database". United States Department of Energy. Retrieved 3 March 2013.
- ^ "Rembrandt" (2 August 2012). "China's Coal to Chemical Future" (Blog post by expert). The Oil Drum.Com. Retrieved 3 March 2013.
- ^ Ken Yin (27 February 2012). "China develops coal-to-olefins projects, which could lead to ethylene self-sufficiency". ICIS Chemical Business. Retrieved 3 March 2013.
- ^ Didi Kirsten Tatlow (5 February 2013). "Worse Than Poisoned Water: Dwindling Water, in China's North" (Blog in edited newspaper). International Herald Tribune. Retrieved 3 March 2013.
- ^ "Spill in China Underlines Environmental Concerns". The New York Times. 2 March 2013. Retrieved 3 March 2013.
- ^ "Kentucky: Secretary of State – State Mineral". 20 October 2009. Retrieved 7 August 2011.
- ^ "Utah State Rock – Coal". Pioneer: Utah's Online Library. Utah State Library Division. Retrieved 7 August 2011.
- ^ "NYMEX.com: Coal". Archived from the original on 12 March 2008. Retrieved 16 January 2008.
- ^ "ICE: Coal Futures". Retrieved 16 January 2008.
- ^ Coal News and Markets (Archive) Department of Energy – see Bloomberg for realtime prices
- ^ Toxic Air: The Case for Cleaning Up Coal-fired Power Plants. American Lung Association (March 2011)
- ^ Environmental impacts of coal power: air pollution. Union of Concerned Scientists
- ^ "Deadly power plants? Study Fuels Debate: Thousands of Early Deaths Tied To Emissions." MSNBC (2004-09-06) Retrieved 5 November 2008
- ^ "The Unpaid Health Bill – How coal power plants make us sick". Health and Environment Alliance. Retrieved 7 March 2013.
- ^ World Coal Association "Environmental impact of Coal Use"
- ^ Direct Testimony of James E. Hansen. State of Iowa
- ^ Human Impacts on Atmospheric Chemistry, by PJ Crutzen and J Lelieveld, Annual Review of Earth and Planetary Sciences, Vol. 29: 17 -45 (Volume publication date May 2001)
- ^ J. A. Campbell; D. L. Stewart; M. McCulloch; R. B. Lucke; R. M. Bean. "BIODEGRADATION OF COAL-RELAED MODEL COMPOUNDS" (PDF). Pacific Northwest Laboratory: 514–521.
{{cite journal}}: Cite journal requires|journal=(help) - ^ M.C. Potter (May 1908). "Bateria as agents in the oxidation of amorphous carbon". Proceedings of the Royal Society of London Series B, Containing Papers of a Biological Character. 80: 239–259.
- ^ Peckham, Jack (2002). "Diesel Fuel News: Ultra-clean fuels from coal liquefaction: China about to launch big projects – Brief Article". Diesel Fuel News. Retrieved 9 September 2005.
- ^ Tingting, Si and Jing, Li (12 December 2008). "Coal-to-liquids project rescheduled to launch in early 2009". China Daily. Retrieved 12 December 2008.
{{cite news}}: CS1 maint: multiple names: authors list (link) - ^ "Sasol, Shenhua Group May Complete Coal-to-Fuel Plant by 2013". Bloomberg. 7 January 2009. Retrieved 10 January 2009.
- ^ "China". United States Energy Information Administration.
- ^ Elegant, Simon; Zhangjiachang (2 March 2007). "Where The Coal Is Stained With Blood". TIME. Retrieved 25 July 2014.
- ^ "The human cost of coal in the UK: 1600 deaths a year". New Scientist.
- ^ http://www.economist.com/blogs/freeexchange/2014/02/environmentalism
- ^ Fisher, Juliya (2003). "Energy Density of Coal". The Physics Factbook. Retrieved 25 August 2006.
- ^ "How much coal is required to run a 100-watt light bulb 24 hours a day for a year?". Howstuffworks. Retrieved 25 August 2006.
- ^ Analysis: Efficiency of coal-fired power stations – evolution and prospects. EurActiv. Retrieved on 24 August 2012.
- ^ CO2 Carbon Dioxide Emissions from the Generation of Electric Power in the United States, DOE, EPA, 1999. Eia.doe.gov. Retrieved on 24 August 2012.
- ^ "Sino German Coal fire project". Retrieved 9 September 2005.
- ^ "Committee on Resources-Index". Archived from the original on 25 August 2005. Retrieved 9 September 2005.
- ^ "Snapshots 2003" (PDF). fire.blm.gov. Archived from the original (PDF) on 18 February 2006. Retrieved 9 September 2005.
- ^ "EHP 110-5, 2002: Forum". Retrieved 9 September 2005.
- ^ "Overview about ITC's activities in China". Archived from the original on 16 June 2005. Retrieved 9 September 2005.
- ^ "Fire in The Hole". Retrieved 5 June 2011.
- ^ "North Dakota's Clinker". Retrieved 9 September 2005.
- ^ "BLM-Environmental Education- The High Plains". Archived from the original on 12 March 2005. Retrieved 9 September 2005.
- ^ Lyman, Robert M. and Volkmer, John E. (March 2001). "Pyrophoricity (spontaneous combustion) of Powder River Basin coals: Considerations for coalbed methane development" (PDF). Archived from the original (PDF) on 12 September 2005. Retrieved 9 September 2005.
{{cite web}}: C1 control character in|title=at position 67 (help)CS1 maint: multiple names: authors list (link) - ^ "EIA projects little change in U.S. coal production in 2013". U.S. Energy Information Agency. 3 December 2012. Retrieved 20 December 2012.
- ^ Lipton, Eric (19 December 2012). "Power Company Loses Some of Its Appetite for Coal". The New York Times. Retrieved 20 December 2012.
- ^ Le, Phuong (25 March 2013). "NW governors ask White House to exam coal exports". The San Francisco Chronicle. Associated Press. Retrieved 26 March 2013.
- ^ Clifford Krauss (14 June 2013). "Coal Industry Pins Hopes on Exports as U.S. Market Shrinks". The New York Times. Retrieved 15 June 2013.
- ^ Suhr, Jim (1 May 2013). "Report: Ill. coal enjoyed record exports in 2012". San Francisco Chronicle. Associated Press. Retrieved 2 May 2013.
- ^ "EIA International Energy Statistics : Coal : Consumption". Retrieved 10 June 2009.
- ^ "Coal: Resources and Future Production" (PDF). Energy Watch Group. 10 July 2007. Archived from the original (PDF) on 5 July 2010.
- ^ World Energy Council – Survey of Energy Resources 2010. (PDF) . Retrieved on 24 August 2012.
- ^ Sherwood, Alan and Phillips, Jock. Coal and coal mining – Coal resources, Te Ara – the Encyclopedia of New Zealand, updated 2009-03-02
- ^ EIA International Energy Annual – Total Coal Consumption (Thousand Short Tons). Eia.gov. Retrieved on 2013-05-11.
- ^ Table 114. World Metallurgical Coal Flows By Importing Regions and Exporting Countries 1,2/ (million short tons). eia.doe.gov
- ^ World Coal Flows by Importing and Exporting Regions. Eia.doe.gov. Retrieved on 24 August 2012.
- ^ EIA International Energy Annual: Coal Overview 2010. Eia.gov. Retrieved on 24 August 2012.
Further reading
- Walter Licht, Thomas Dublin (2005). The Face of Decline: The Pennsylvania Anthracite Region in the Twentieth Century. Cornell University Press. ISBN 0-8014-8473-1. OCLC 60558740.
- Long, Priscilla (1991). Where the Sun Never Shines: A History of America's Bloody Coal Industry. New York, NY: Paragon House. ISBN 1-55778-465-5. OCLC 25236866.
- Rottenberg, Dan (2003). In the Kingdom of Coal; An American Family and the Rock That Changed the World. Routledge. ISBN 0-415-93522-9. OCLC 52348860.
- Robert H. Williams and Eric D. Larson (December 2003). "A comparison of direct and indirect liquefaction technologies for making fluid fuels from coal" (PDF). Energy for Sustainable Development. VII: 103–129.
- Outwater, Alice (1996). Water: A Natural History. New York, NY: Basic Books. ISBN 0-465-03780-1. OCLC 37785911.
- Smith, Duane A. (May 1993). Mining America: The Industry and the Environment, 1800–1980. Lawrence, KS: University Press of Kansas. p. 210. ISBN 0-87081-306-4.
- Freese, Barbara (2003). Coal: A Human History. Penguin Books. ISBN 0-7382-0400-5. OCLC 51449422.
External links
- Coal news and industry magazine
- Coal Online – International Energy Agency
- Coal Research at the National Energy Technology Laboratory
- Energy KIDS – Coal page from U. S. Department of Energy.
- European Association for Coal and Lignite
- World Coal Association
- Coal: Facts & Figures
- International Coal Price Data


