Degrees of freedom (physics and chemistry)
- For information on degrees of freedom in other sciences, see degrees of freedom. For other uses of degree, see Degree
Degrees of freedom is a general term used in explaining dependence on parameters, and implying the possibility of counting the number of those parameters.
Degrees of freedom in mechanics (physics)
In mechanics, for each particle belonging to a system, and for each independent direction in which movement is possible, two degrees of freedom are defined, one describing the particle's momentum in that direction, the other describing the particle's position along an axis defined by that direction.
Note that "degrees of freedom" has a different meaning in the context of engineering and machines.
A more general definition
In statistical mechanics, a degree of freedom is a single scalar number describing the classical micro-state of a system. The micro-state of a system is completely described by the set of all values of all its degrees of freedom.
If the system studied can be described as a set of mechanical particles, then degrees of freedom are defined in the same manner as above. Thus, a micro-state of the system is a point in the system's phase space.
It must be noted that for a system, a micro-state defined by using degrees of freedom is intrinsically a classical state. This is because for a quantum micro-state, defining a precise value of both the position and momentum of a particle violates the Heisenberg uncertainty principle. The description of a system through a set of degrees of freedom is thus only valid in the classical (or high temperature) limit of statistical mechanics.
In some cases, when the system is not appropriately described as a set of mechanical particles, other types of degrees of freedom have to be defined. For example, in the 3D ideal chain model, two angles are necessary to describe each monomer's orientation. The value of each of these angles can each be a degree of freedom.
Example: classical ideal diatomic gas
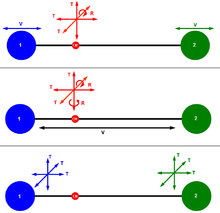
In 3D, there are 6 degrees of freedom associated to the movement of a mechanical particle, 3 for its position, and 3 for its momentum.
There are 6 degrees of freedom in total. Another way to justify this figure is to consider that the movement of the molecule will be described by the movement of the two mechanical particles representing its two atoms, and 6 degrees of freedom are attached to each particle, as above. With this alternative breakdown, it appears that different sets of degrees of freedom can be defined to describe the movement of the molecule. In fact a set of degrees of freedom for a mechanical system is a set of independent axes in the phase space of the system, and that allows the generation of the whole phase space. For a multidimensional space like phase space, there is more than one possible set of axes.
It is notable that not all degrees of freedom of the hydrogen molecule participate in the above expression of its energy. For example, those degrees of freedom associated to the position of the center of mass of the particle do not weigh in the energy.
In the table below the degrees which are disregarded are like this because of their low effect on total energy, unless they are at very very high temperatures or energies. The diatomic rotation is disregarded due to rotation about the molecules axis. Monatomic rotation is disregarded for the same reason as diatomic, but this effect continues into the other 2 directions.
| Monatomic | Linear molecules | Non-Linear molecules | |
|---|---|---|---|
| Position (x, y and z) | 3 | 3 | 3 |
| Rotation (x, y and z) | 0 | 2 | 3 |
| Vibration | 0 | 3N - 5 | 3N - 6 |
| Total | 3 | 3N | 3N |
Independent degrees of freedom
Definition
The set of degrees of freedom of a system is independent if the energy associated with the set can be written in the following form:
where is a function of the sole variable .
example: if and are two degrees of freedom, and is the associated energy:
- If , then the two degrees of freedom are independent.
- If , then the two degrees of freedom are not independent. The term involving the product of and is a coupling term, that describes an interaction between the two degrees of freedom.
Properties
If is a set of independent degrees of freedom then, at thermodynamic equilibrium, are all statistically independent from each other.
For i from 1 to N, the value of the ith degree of freedom is distributed according to the Boltzmann distribution. Its probability density function is the following:
- ,
In this section, and throughout the article the brackets denote the mean of the quantity they enclose.
The internal energy of the system is the sum of the average energies associated to each of the degrees of freedom:
Demonstrations
We will assume that our system exchanges energy in the form of heat with the outside, and that its number of particles remains fixed. This corresponds to studying the system in the canonical ensemble. Note that in statistical mechanics, a result that is demonstrated for a system in a particular ensemble remains true for this system at the thermodynamic limit in any ensemble. In the canonical ensemble, at thermodynamic equilibrium, the state of the system is distributed among all micro-states according to the Boltzmann distribution. If is the system's temperature and is Boltzman's constant, then the probability density function associated to each micro-state is the following:
- ,
This expression immediately breaks down into a product of terms depending of a single degree of freedom:
The existence of such a breakdown of the multidimensional probability density function into a product of functions of one variable is enough by itself to demonstrate that are statistically independent from each other.
Since each function is normalized, it follows immediately that is the probability density function of the degree of freedom , for i from 1 to N.
Finally, the internal energy of the system is its mean energy. The energy of a degree of freedom is a function of the sole variable . Since are independent from each other, the energies are also statistically independent from each other. The total internal energy of the system can thus be written as:
Quadratic degrees of freedom
A degree of freedom is quadratic if the energy terms associated to this degree of freedom can be written as:
- ,
where is a linear combination of other quadratic degrees of freedom.
example: if and are two degrees of freedom, and is the associated energy:
- If , then the two degrees of freedom are not independent and non-quadratic.
- If , then the two degrees of freedom are independent and non-quadratic.
- If , then the two degrees of freedom are not independent but are quadratic.
- If , then the two degrees of freedom are independent and quadratic.
Quadratic degrees of freedom in mechanics
In Newtonian mechanics, the dynamics of a system of quadratic degrees of freedom are controlled by a set of homogeneous linear differential equations with constant coefficients.
Quadratic and independent degree of freedom
are quadratic and independent degrees of freedom if the energy associated to a microstate of the system they represent can be written as:
In the classical limit of statistical mechanics, at thermodynamic equilibrium, the internal energy of a system of N quadratic and independent degrees of freedom is:
Demonstration
Here, the mean energy associated with a degree of freedom is:
Since the degrees of freedom are independent, the internal energy of the system is equal to the sum of the mean energy associated to each degree of freedom, which demonstrates the result.
See also
- Entropy, in which the concept of micro-state is introduced
- Phase space
- Statistical mechanics






























