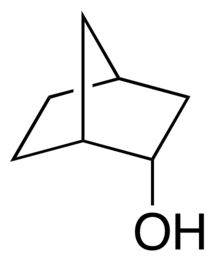
endo-Norborneol
Appearance
| |
| Names | |
|---|---|
| IUPAC names
(1R,2S,4S)-Bicyclo[2.2.1]heptan-2-ol
(1S,2R,4R)-Bicyclo[2.2.1]heptan-2-ol | |
| Other names
endo-2-Norborneol; endo-Norbornyl alcohol
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.127.627 |
PubChem CID
|
|
| |
| |
| Properties | |
| C7H12O | |
| Molar mass | 112.17 g/mol |
| Melting point | 149 to 154 °C (300 to 309 °F; 422 to 427 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
endo-Norborneol is an alcohol.
See also
References
External links
- endo-Norborneol MSDS
- Reaction of organic compounds under high temperature – dilute acid (HTDA) conditions. III. The perdeuteration of bicyclo[2.2.1]heptanes PDF
- J. K. Stille, and Fred M. Sonnenberg (1966). "The Reaction of endo- and exo-2-Norborneol with Thionyl Chloride" (PDF). J. Am. Chem. Soc. 88 (21): 4915–4921. doi:10.1021/ja00973a027.
