Iron(III) acetate
| |
| |
| Names | |
|---|---|
| IUPAC name
iron(III) acetate
| |
| Other names
basic iron(III) acetate , iron(III) oxyacetate, iron(III) Acetate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H27Fe3O18 | |
| Molar mass | 650.9 g/mol |
| Appearance | brownish-red powder |
| Insoluble | |
| Solubility | soluble in ethanol[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
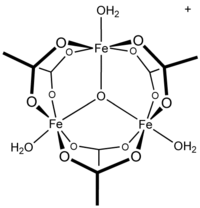
Ferric acetate is the acetate salt of the coordination complex [Fe3O(OAc)6(H2O)3]+ (OAc− is CH3CO2−). Commonly the salt is known as "basic iron acetate".[3] The formation of the red-brown complex was once used as a test for ferric ions.[4]
Structure and synthesis[edit]
Basic iron acetate forms on treating aqueous solutions of iron(III) sources with acetate salts.[5] A typical precursor is freshly precipitated iron oxide/hydroxide, which is halide-free.[6]
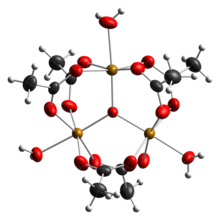
Early work showed that the cation is trinuclear.[7] The Fe centres are equivalent, each being octahedral, being bound to six oxygen ligands, including a triply bridging oxide at the center of the equilateral triangle.[8] The compound was an early example of a molecular complex of iron that features an oxide ligand. The cation has idealized D3h symmetry.
Reactions[edit]
The terminal aqua ligands on the trimetallic framework can be substituted with other ligands, such as pyridine and dimethylformamide. Many different salts are known by exchanging the anion, e.g. [Fe3(μ3-O)(OAc)6(H2O)3]Cl. Reduction of the cation affords the neutral mixed-valence derivative that contains one ferrous and two ferric centers.[3] Mixed metal species are known such as [Fe2CoO(OAc)6(H2O)3].[9]
Related compounds[edit]
Chromium(III), ruthenium(III), vanadium(III), and rhodium(III) form analogous compounds.[10] Iron(III) acetate (lacking the oxo ligand) has been claimed as a red coloured compound from the reaction of silver acetate and iron(III) chloride.[11]
Uses[edit]
Materials prepared by heating iron, acetic acid, and air, loosely described as basic iron acetates, are used as dyes and mordants.[3]
Iron acetate is often brushed upon untreated wood to give it an aged appearance.
See also[edit]
- Iron(II) acetate
- Cobalt(II) acetate
- Rhodium(II) acetate
- Manganese(III) acetate
- Chromium(III) acetate
References[edit]
- ^ Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. pp. 4–63. ISBN 0-8493-0487-3.
- ^ "Iron(III) Acetate". EndMemo. Retrieved 18 April 2015.
- ^ a b c J., Burgess; M. V., Twigg (2005). R. Bruce, King; J., Wiley (eds.). Encyclopedia of inorganic chemistry (2nd ed.). New York: Wiley. ISBN 978-0-470-86078-6.
- ^ H., Brearley; F., Ibbotson (1902). The Analysis of Steel-Works Materials. London ; New York: Longmans, Green. Archived from the original on 18 April 2015.
- ^ W., Simon (March 2007). Manual of Chemistry. p. 474. ISBN 978-1406733358.
- ^ H. Lux (1963). "Basic Iron(III) Acetate". In Georg Brauer (ed.). Handbook of Preparative Inorganic Chemistry. p. 1508.
- ^ Weinland, R.; Dinkelacker, P. (July 1909). "Über Salze einer Hexaacetato(formiato)-trichrombase. II". Berichte der Deutschen Chemischen Gesellschaft. 42 (3): 2997–3018. doi:10.1002/cber.19090420318.
- ^ Figgis, B. N.; Robertson, G. B. (13 February 1965). "Crystal-Molecular Structure and Magnetic Properties of Cr3(CH3.COO)6OCl.5H2O". Nature. 205 (4972): 694–695. Bibcode:1965Natur.205..694F. doi:10.1038/205694a0. S2CID 4283321. This paper describes the isostructure chromium and iron compounds.
- ^ Blake, Antony B.; Yavari, Ahmad; Hatfield, William E.; Sethulekshmi, C. N. (1985). "Magnetic and spectroscopic properties of some heterotrinuclear basic acetates of chromium(III), iron(III), and divalent metal ions". Journal of the Chemical Society, Dalton Transactions (12): 2509. doi:10.1039/DT9850002509.
- ^ Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, ISBN 0-12-352651-5
- ^ Paul, Ram C.; Narula, Ramesh C.; Vasisht, Sham K. (December 1978). "Iron(III) acetates". Transition Metal Chemistry. 3 (1): 35–38. doi:10.1007/BF01393501. S2CID 94447648.
