Gamma secretase
| Gamma-secretase (Nicastrin subunit) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
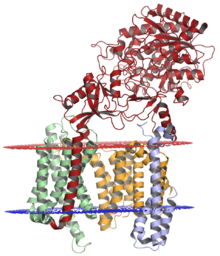 The gamma secretase complex, with nicastrin (red), presenilin-1 (orange), PEN-2 (blue), and APH-1 (green); lumenal membrane shown in red and cytoplasmic membrane shown in blue. The structure was solved by cryo-electron microscopy.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Gamma-secretase, γ-secretase | ||||||||
| Pfam | PF05450 | ||||||||
| InterPro | IPR008710 | ||||||||
| OPM superfamily | 244 | ||||||||
| OPM protein | [ 5fn5[ | ||||||||
| Membranome | 155 | ||||||||
| |||||||||
Gamma secretase is a multi-subunit protease complex, itself an integral membrane protein, that cleaves single-pass transmembrane proteins at residues within the transmembrane domain. Proteases of this type are known as intramembrane proteases. The most well-known substrate of gamma secretase is amyloid precursor protein, a large integral membrane protein that, when cleaved by both gamma and beta secretase, produces a short 37-43[verification needed] amino acid peptide called amyloid beta whose abnormally folded fibrillar form is the primary component of amyloid plaques found in the brains of Alzheimer's disease patients. Gamma secretase is also critical in the related processing of several other type I integral membrane proteins, such as Notch,[2] ErbB4,[3] E-cadherin,[4] N-cadherin,[5] ephrin-B2,[6] or CD44.[7]
Subunits and assembly[edit]
The gamma secretase complex consists of four individual proteins: PSEN1 (presenilin-1),[8] nicastrin, APH-1 (anterior pharynx-defective 1), and PEN-2 (presenilin enhancer 2).[9] Recent evidence suggests that a fifth protein, known as CD147, is a non-essential regulator of the complex whose absence increases activity.[10][11] Presenilin, an aspartyl protease, is the catalytic subunit; mutations in the presenilin gene have been shown to be a major genetic risk factor for Alzheimer's disease [12] and modulates immune cell activity.[13] In humans, two forms of presenilin and two forms of APH-1 have been identified in the genome; one of the APH homologs can also be expressed in two isoforms via alternative splicing, leading to at least six different possible gamma secretase complexes that may have tissue- or cell type specificity.[14]
The proteins in the gamma secretase complex are heavily modified by proteolysis during assembly and maturation of the complex; a required activation step is in the autocatalytic cleavage of presenilin to N- and C-terminal fragments. Nicastrin's primary role is in maintaining the stability of the assembled complex and regulating intracellular protein trafficking.[15] PEN-2 associates with the complex via binding of a transmembrane domain of presenilin[16] and, among other possible roles, helps to stabilize the complex after presenilin proteolysis has generated the activated N-terminal and C-terminal fragments.[17] APH-1, which is required for proteolytic activity, binds to the complex via a conserved alpha helix interaction motif and aids in initiating assembly of premature components.[18]
Recent research has shown that interaction of the gamma secretase complex with the γ-secretase activating protein facilitates the gamma cleavage of amyloid precursor protein into β-amyloid.[19]
Cellular trafficking[edit]
The gamma secretase complex is thought to assemble and mature via proteolysis in the early endoplasmic reticulum.[20] The complexes are then transported to the late ER where they interact with and cleave their substrate proteins.[21] Gamma secretase complexes have also been observed localized to the mitochondria, where they may play a role in promoting apoptosis.[22]
Function[edit]
Gamma secretase is an internal protease that cleaves within the membrane-spanning domain of its substrate proteins, including amyloid precursor protein (APP) and Notch. Substrate recognition occurs via nicastrin ectodomain binding to the N-terminus of the target, which is then passed via a poorly understood process between the two presenilin fragments to a water-containing active site where the catalytic aspartate residue is located. The active site must contain water to carry out hydrolysis within a hydrophobic environment in the interior of the cell membrane, although it is not well understood how water and proton exchange is effected, and as yet no X-ray crystallography structure of gamma secretase is available.[23] Low-resolution electron microscopy reconstructions have allowed the visualization of the hypothesized internal pores of about 2 nanometres.[24] In 2014, a three-dimensional structure of an intact human gamma-secretase complex was determined by cryo-electron microscopy single-particle analysis at 4.5 angstrom resolution[25] and in 2015 an atomic-resolution (3.4 angstrom) cryo-EM structure was reported.[1]
The gamma secretase complex is unusual among proteases in having a "sloppy" cleavage site at the C-terminal site in amyloid beta generation; gamma secretase can cleave APP in any of multiple sites to generate a peptide of variable length, most typically from 39 to 42 amino acids long, with Aβ40 the most common isoform and Aβ42 the most susceptible to conformational changes leading to amyloid fibrillogenesis. Certain mutations in both APP and in both types of human presenilin are associated with increased Aβ42 production and the early-onset genetic form of familial Alzheimer's disease.[26] Although older data suggested that different forms of the gamma secretase complex could be differentially responsible for generating different amyloid beta isoforms,[27] current evidence indicates that the C-terminus of amyloid beta is produced by a series of single-residue cleavages by the same gamma secretase complex.[28][29][30] Earlier cleavage sites produce peptides of length 46 (zeta-cleavage) and 49 (epsilon-cleavage).[29]
See also[edit]
- DAPT (chemical), a γ-secretase inhibitor
References[edit]
- ^ a b Bai, Xiao-chen; Yan, Chuangye; Yang, Guanghui; Lu, Peilong; Ma, Dan; Sun, Linfeng; Zhou, Rui; Scheres, Sjors H. W.; Shi, Yigong (17 August 2015). "An atomic structure of human γ-secretase". Nature. 525 (7568): 212–217. doi:10.1038/nature14892. PMC 4568306. PMID 26280335.
- ^ De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R (1999). "A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain". Nature. 398 (6727): 518–22. doi:10.1038/19083. PMID 10206645. S2CID 4346474.
- ^ Ni CY, Murphy MP, Golde TE, Carpenter G (2001). "gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase". Science. 294 (5549): 2179–81. doi:10.1126/science.1065412. PMID 11679632. S2CID 23227013.
- ^ Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, Wisniewski T, Robakis NK (2002). "A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions". EMBO J. 21 (8): 1948–56. doi:10.1093/emboj/21.8.1948. PMC 125968. PMID 11953314.
- ^ Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, Robakis NK (2003). "A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations". Cell. 114 (5): 635–45. doi:10.1016/j.cell.2003.08.008. PMID 13678586. S2CID 7265454.
- ^ Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G, Robakis NK (2006). "Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling". EMBO J. 25 (6): 1242–52. doi:10.1038/sj.emboj.7601031. PMC 1422162. PMID 16511561.
- ^ Lammich S, Okochi M, Takeda M, Kaether C, Capell A, Zimmer AK, Edbauer D, Walter J, Steiner H, Haass C (2002). "Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an Abeta-like peptide". J Biol Chem. 277 (47): 44754–9. doi:10.1074/jbc.M206872200. PMID 12223485.
- ^ Sobhanifar, S; Schneider, B; Löhr, F; Gottstein, D; Ikeya, T; Mlynarczyk, K; Pulawski, W; Ghoshdastider, U; Kolinski, M; Filipek, S; Güntert, P; Bernhard, F; Dötsch, V (25 May 2010). "Structural investigation of the C-terminal catalytic fragment of presenilin 1". Proceedings of the National Academy of Sciences of the United States of America. 107 (21): 9644–9. doi:10.1073/pnas.1000778107. PMC 2906861. PMID 20445084.
- ^ Kaether C, Haass C, Steiner H (2006). "Assembly, trafficking and function of gamma-secretase" (PDF). Neurodegener Dis. 3 (4–5): 275–83. doi:10.1159/000095267. PMID 17047368. S2CID 17324271.
- ^ Zhou S, Zhou H, Walian PJ, Jap BK (April 2006). "The discovery and role of CD147 as a subunit of gamma-secretase complex". Drug News Perspect. 19 (3): 133–8. doi:10.1358/dnp.2006.19.3.985932. PMID 16804564.
- ^ Zhou S, Zhou H, Walian PJ, Jap BK (May 2005). "CD147 is a regulatory subunit of the γ-secretase complex in Alzheimer's disease amyloid β-peptide production". Proc. Natl. Acad. Sci. U.S.A. 102 (21): 7499–504. doi:10.1073/pnas.0502768102. PMC 1103709. PMID 15890777.
- ^ Chen F, Hasegawa H, Schmitt-Ulms G, Kawarai T, Bohm C, Katayama T, Gu Y, Sanjo N, Glista M, Rogaeva E, Wakutani Y, Pardossi-Piquard R, Ruan X, Tandon A, Checler F, Marambaud P, Hansen K, Westaway D, St George-Hyslop P, Fraser P (April 2006). "TMP21 is a presenilin complex component that modulates gamma-secretase but not epsilon-secretase activity". Nature. 440 (7088): 1208–12. doi:10.1038/nature04667. PMID 16641999. S2CID 4349251.
- ^ Farfara D, Trudler D, Segev-Amzaled N, Galron R, Stein R, Frenkel D (November 2010). "g secretase component presenilin is important for microglia b-Amyloid clearance". Annals of Neurology. 69 (1): 170–80. doi:10.1002/ana.22191. PMID 21280087. S2CID 20603724.
- ^ Shirotani K, Edbauer D, Prokop S, Haass C, Steiner H (2004). "Identification of distinct gamma-secretase complexes with different APH-1 variants". J Biol Chem. 279 (40): 41340–5. doi:10.1074/jbc.M405768200. PMID 15286082.
- ^ Zhang YW, Luo WJ, Wang H, Lin P, Vetrivel KS, Liao F, Li F, Wong PC, Farquhar MG, Thinakaran G, Xu H (April 2005). "Nicastrin Is Critical for Stability and Trafficking but Not Association of Other Presenilin/γ-Secretase Components". J. Biol. Chem. 280 (17): 17020–6. doi:10.1074/jbc.M409467200. PMC 1201533. PMID 15711015.
- ^ Watanabe N, Tomita T, Sato C, Kitamura T, Morohashi Y, Iwatsubo T (December 2005). "Pen-2 is incorporated into the gamma-secretase complex through binding to transmembrane domain 4 of presenilin 1". J. Biol. Chem. 280 (51): 41967–75. doi:10.1074/jbc.M509066200. PMID 16234244.
- ^ Prokop S, Shirotani K, Edbauer D, Haass C, Steiner H (May 2004). "Requirement of PEN-2 for stabilization of the presenilin N-/C-terminal fragment heterodimer within the gamma-secretase complex". J. Biol. Chem. 279 (22): 23255–61. doi:10.1074/jbc.M401789200. PMID 15039426.
- ^ Lee SF, Shah S, Yu C, Wigley WC, Li H, Lim M, Pedersen K, Han W, Thomas P, Lundkvist J, Hao YH, Yu G (February 2004). "A conserved GXXXG motif in APH-1 is critical for assembly and activity of the gamma-secretase complex". J. Biol. Chem. 279 (6): 4144–52. doi:10.1074/jbc.M309745200. PMID 14627705.
- ^ He G, Luo W, Li P, Remmers C, Netzer WJ, Hendrick J, Bettayeb K, Flajolet M, Gorelick F, Wennogle LP, Greengard P (September 2010). "Gamma-secretase activating protein, a therapeutic target for Alzheimer's disease". Nature. 467 (2): 95–98. doi:10.1038/nature09325. PMC 2936959. PMID 20811458.
- Gina Kolata (September 1, 2010). "Finding Suggests New Aim for Alzheimer's Drugs". The New York Times.
- ^ Capell A, Beher D, Prokop S, Steiner H, Kaether C, Shearman MS, Haass C (February 2005). "Gamma-secretase complex assembly within the early secretory pathway". J. Biol. Chem. 280 (8): 6471–8. doi:10.1074/jbc.M409106200. PMID 15591316.
- ^ Kim SH, Yin YI, Li YM, Sisodia SS (November 2004). "Evidence that assembly of an active gamma-secretase complex occurs in the early compartments of the secretory pathway". J. Biol. Chem. 279 (47): 48615–9. doi:10.1074/jbc.C400396200. PMID 15456788.
- ^ Hansson CA, Frykman S, Farmery MR, Tjernberg LO, Nilsberth C, Pursglove SE, Ito A, Winblad B, Cowburn RF, Thyberg J, Ankarcrona M (December 2004). "Nicastrin, presenilin, APH-1, and PEN-2 form active gamma-secretase complexes in mitochondria". J. Biol. Chem. 279 (49): 51654–60. doi:10.1074/jbc.M404500200. PMID 15456764.
- ^ Wolfe MS (July 2006). "The gamma-secretase complex: membrane-embedded proteolytic ensemble". Biochemistry. 45 (26): 7931–9. doi:10.1021/bi060799c. PMID 16800619.
- ^ Lazarov VK, Fraering PC, Ye W, Wolfe MS, Selkoe DJ, Li H (May 2006). "Electron microscopic structure of purified, active γ-secretase reveals an aqueous intramembrane chamber and two pores". Proc. Natl. Acad. Sci. U.S.A. 103 (18): 6889–94. doi:10.1073/pnas.0602321103. PMC 1458989. PMID 16636269.
- ^ Lu P, Bai XC, Ma D, Xie T, Yan C, Sun L, Yang G, Zhao Y, Zhou R, Scheres SH, Shi Y (August 2014). "Three-dimensional structure of human γ-secretase". Nature. 512 (7513): 166–170. doi:10.1038/nature13567. PMC 4134323. PMID 25043039.
- ^ Wiley JC, Hudson M, Kanning KC, Schecterson LC, Bothwell M (September 2005). "Familial Alzheimer's disease mutations inhibit gamma-secretase-mediated liberation of beta-amyloid precursor protein carboxy-terminal fragment". J. Neurochem. 94 (5): 1189–201. doi:10.1111/j.1471-4159.2005.03266.x. PMID 15992373.
- ^ Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR (January 2004). "Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase". Hum. Mol. Genet. 13 (2): 159–70. doi:10.1093/hmg/ddh019. PMID 14645205.
- ^ Zhao G, Tan J, Mao G, Cui MZ, Xu X (March 2007). "The same gamma-secretase accounts for the multiple intramembrane cleavages of APP". J. Neurochem. 100 (5): 1234–46. doi:10.1111/j.1471-4159.2006.04302.x. PMID 17241131.
- ^ a b Zhang, H; Ma, Q; Zhang, YW; Xu, H (January 2012). "Proteolytic processing of Alzheimer's β-amyloid precursor protein". Journal of Neurochemistry. 120 Suppl 1: 9–21. doi:10.1111/j.1471-4159.2011.07519.x. PMC 3254787. PMID 22122372.
- ^ Haass, C; Kaether, C; Thinakaran, G; Sisodia, S (May 2012). "Trafficking and proteolytic processing of APP". Cold Spring Harbor Perspectives in Medicine. 2 (5): a006270. doi:10.1101/cshperspect.a006270. PMC 3331683. PMID 22553493.
