Julia olefination
The Julia olefination (also known as the Julia–Lythgoe olefination) is the chemical reaction of phenyl sulfones (1) with aldehydes (or ketones) to give alkenes (3) after alcohol functionalization and reductive elimination using sodium amalgam[1][2] or SmI2.[3] The reaction is named after the French chemist Marc Julia.
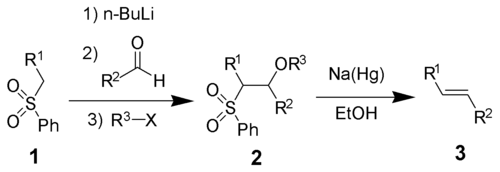
This transformation highly favors formation of the trans-alkene.
All four steps can be carried out in a single reaction vessel, and use of R3X is optional. However, purification of the sulfone intermediate 2 leads to higher yield and purity. Most often R3 is acetyl or benzoyl, with acetic anhydride or benzoyl chloride used in the preparation of 2.
Several reviews have been published.[4][5]
Reaction mechanism
The initial steps are straightforward. The phenyl sulfone anion (2) reacts with an aldehyde to form the alkoxide 3. The alkoxide is functionalized with R3-X to give the stable intermediate 4. The exact mechanism of the sodium amalgam reduction is unknown but has been shown to proceed through a vinylic radical species (5).[1] Protonation of the vinylic radical give the desired product (6).
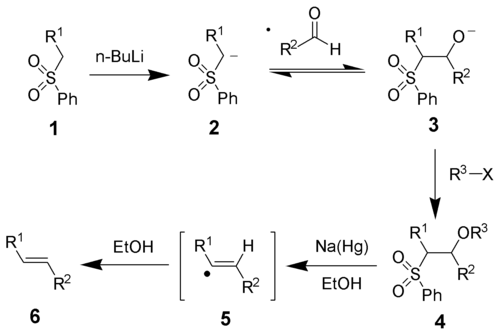
The stereochemistry of the alkene (6) is independent of the stereochemistry of the sulfone intermediate 4. It is thought that the radical intermediates are able to equilibrate so that the more thermodynamically stable trans-olefin is produced most often.
Variations
Heteroaryl sulfones
The replacement of the phenyl sulfones with heteroaryl sulfones greatly alters the reaction pathway.[6] The most popular example is the benzothiazole sulfone.[7] The reaction of the benzothiazole sulfone (1) with lithium diisopropylamide (LDA) gives a metallated benzothiazolyl sulfone, which reacts quickly with aldehydes (or ketones) to give an alkoxides intermediate (2). Unlike the phenyl sulfones, this alkoxide intermediate (2) is unstable and will undergo a Smiles rearrangement[8] to give the sulfinate salt 4. The sulfinate salt (4) will spontaneously eliminate sulfur dioxide and lithium benzothiazolone (5) producing the desired alkene (6).

Since the benzothiazole variation of the Julia olefination does not involve equilibrating intermediates, the stereochemical outcome is a result of the stereochemistry of the initial carbonyl addition. As a result, this reaction often generates a mixture of alkene stereoisomers.
Julia-Kocienski olefination
In the Julia-Kocienski olefination[9] the alkylating agent is a tetrazole. It proceeds with the same mechanism as the benzothiazole sulfone above. In one adaptation,[10] with t-butyltetrazoylmethyl sulfone the reaction conditions are either sodium bis(trimethylsilyl)amide at -70°C in tetrahydrofuran or caesium carbonate at +70°C.

References
- ^ [3]
- ^ Julia, M.; Paris, J.-M. Tetrahedron Lett. 1973, 14, 4833-4836. (doi:10.1016/S0040-4039(01)87348-2)
- ^ Kocienski, P. J.; Lythgoe, B.; Ruston, S. J. Chem. Soc., Perkin Trans. 1 1978, 829.
- ^ Keck, G. E.; Savin, K. A.; Weglarz, M. A. J. Org. Chem. 1995, 60, 3194-3204. (doi:10.1021/jo00115a041)
- ^ Kocienski, P. J. Phosphorus and Sulfur 1985, 24, 97-127. (Review)
- ^ Kelly, S. E. Comp. Org. Syn. 1991, 1, 792-806. (Review)
- ^ Blakemore, P. R. J. Chem. Soc., Perkin Trans. 1 2002, 2563–2585. (Review)
- ^ Baudin, J. B.; Hareau, G.; Julia, S. A.; Ruel, O. Tetrahedron Lett. 1991, 32, 1175. (doi:10.1016/S0040-4039(00)92037-9)
- ^ Truce, W. E.; Kreider, E. M.; Brand, W. W. Org. React. 1970, 18, 99. (Review)
- ^ Paul R. Blakemore, William J. Cole, Philip J. Kocieński, Andrew Morley Synlett 1998, 26-28. (doi:10.1055/s-1998-1570)
- ^ Christophe Aïssa J. Org. Chem. 2006, 71, 360 - 363. (doi:10.1021/jo051693a)
