List of benzodiazepines
| Benzodiazepines |
|---|
 |
The below tables contain a sample list of benzodiazepines and benzodiazepine analogs that are commonly prescribed, with their basic pharmacological characteristics such as half-life and equivalent doses to other benzodiazepines also listed, along with their trade names and primary uses. The elimination half-life is how long it takes for half of the drug to be eliminated by the body. "Time to peak" refers to when maximum levels of the drug in the blood occur after a given dose. Benzodiazepines generally share the same pharmacological properties, such as anxiolytic, sedative, hypnotic, skeletal muscle relaxant, amnesic and anticonvulsant (hypertension in combination with other antihypertensive medications). Variation in potency of certain effects may exist amongst individual benzodiazepines. Some benzodiazepines produce active metabolites. Active metabolites are produced when a person's body metabolizes the drug into compounds that share a similar pharmacological profile to the parent compound and thus are relevant when calculating how long the pharmacological effects of a drug will last. Long-acting benzodiazepines with long-acting active metabolites such as diazepam and chlordiazepoxide are often prescribed for benzodiazepine or alcohol withdrawal as well as for anxiety if constant dose levels are required throughout the day. Shorter-acting benzodiazepines are often preferred for insomnia due to their lesser hangover effect.[1][2][3][4][5]
It is fairly important to note that elimination half-life of diazepam and chlordiazepoxide as well as other long half-life benzodiazepines is twice as long in the elderly compared to younger individuals. Individuals with an impaired liver also metabolize benzodiazepines more slowly. Many doctors[who?] make the mistake of not adjusting benzodiazepine dosage according to age in elderly patients. Thus, the approximate equivalent of doses below may need to be adjusted accordingly in individuals on short acting benzodiazepines who metabolize long-acting benzodiazepines more slowly and vice versa. The changes are most notable with long acting benzodiazepines as these are prone to significant accumulation in such individuals.[This quote needs a citation] For example, the equivalent dose of diazepam in an elderly individual on lorazepam may be half of what would be expected in a younger individual.[6][7] Equivalencies between individual benzodiazepines can differ by 400 fold on a mg per mg basis; awareness of this fact is necessary for the safe and effective use of benzodiazepines.[8]

Pharmacological properties of common benzodiazepines
Data in the table below is taken from the Ashton "Benzodiazepine Equivalency Table".[9]
This article needs additional citations for verification. (July 2015) |
| Drug Name | Common Brand Names* | Initial Approval Year | Time to Peak (Onset of action in hours) | Elimination Half-Life (h)† [active metabolite] (Average hours and days) | Therapeutic use |
|---|---|---|---|---|---|
| Adinazolam | Deracyn | 1-2 | 3h (=0.12d) | anxiolytic, antidepressant | |
| Alprazolam | Helex, Xanax, Xanor, Onax, Alprox, Restyl, Solanax, Tafil, Neurol | 1981 | 1-2 | 10–20 hours (15h=0.62d) | anxiolytic, antidepressant |
| Bentazepam | Thiadipona | 1-3 | 2–4 hours (3h=0.12d) | anxiolytic | |
| Bretazenil[10] | N/A | ? | 2.5 hours (=0.10d) | anxiolytic, anticonvulsant | |
| Bromazepam | Lectopam, Lexaurin, Lexotanil, Lexotan, Bromam | 1-3 | 20–40 hours (30h=1.25d) | anxiolytic, | |
| Brotizolam | Lendormin, Dormex, Sintonal, Noctilan | 0.5-2 | 4–5 hours (4.5h=0.19d) | hypnotic | |
| Camazepam | Albego, Limpidon, Paxor | 0.5-2 | 6–29 hours (17.5h=0.73d) | anxiolytic | |
| Chlordiazepoxide | Librium, Risolid, Elenium | 1960 | 1.5-4 | 5–30 hours [36–200 hours] (17,5h=0,73d [118h=4.92d]) | anxiolytic |
| Cinazepam | 2-4 | 60h (=2.5d) | hypnotic, anxiolytic | ||
| Cinolazepam | Gerodorm | 0.5-2 | 9 hours (=0.37d) | hypnotic | |
| Clobazam | Onfi, Frisium, Urbanol | 2011 | 1–3 hours | 8–60 hours (34h=1.41d) | anxiolytic, anticonvulsant |
| Clonazepam | Rivatril, Rivotril, Klonopin, Iktorivil, Paxam | 1975 | 1-4 | 19.5–50 hours (34.75h=1.45d) | anticonvulsant, anxiolytic, muscle relaxant |
| Clonazolam | N/A | Research chemical | 0.5-1.5 | 10–18 hours | anxiolytic, anticonvulsant, hypnotic, muscle relaxant |
| Clorazepate | Tranxene, Tranxilium | 1972 | Variable | 32–152 hours (92h=3.83d) | anxiolytic, anticonvulsant |
| Clotiazepam | Veratran, Clozan, Rize | 1-3 | 4 hours (=0.17d) | anxiolytic | |
| Cloxazolam | Sepazon, Olcadil | 2-5 (?) | 80–105 hours (92.5h= 3.85d) | anxiolytic, anticonvulsant | |
| Delorazepam | Dadumir | 1-2 | 80–105 hours (92,5hh=3.85d) | anxiolytic, amnesic | |
| Diazepam | Antenex, Apaurin, Apzepam, Apozepam, Hexalid, Pax, Stesolid, Stedon, Valium, Vival, Valaxona | 1963 | 1-1.5 | 32–47 hours [32–205] (39.5h=1.64d [118.5h=4.94d] | anxiolytic, anticonvulsant, muscle relaxant |
| Diclazepam[11] | Research chemical | 1.5-3 | 220 hours (=7days+) | anxiolytic, amnesic, anticonvulsant, hypnotic, muscle relaxant | |
| Estazolam | ProSom, Nuctalon | 1990 | 1-5 | 10–31 hours (20.5h=0.85d) | hypnotic, anxiolytic |
| Ethyl carfluzepate | N/A | 1-5 | 11–24 hours (17.5h=0.73d) | hypnotic | |
| Etizolam | Etilaam, Etizest, Pasaden, Depas | 1-2 | 6 hours (=0.25d) | anxiolytic, hypnotic, amnesic, muscle relaxant, anticonvulsant | |
| Ethyl loflazepate | Victan, Meilax, Ronlax | 2.5-3 | 73–119 hours (96h=4d) | anxiolytic | |
| Flubromazepam[12] | Research chemical | 1.5-4 (4-8) | 100–220 hours (160h=6.67d) | anxiolytic, hypnotic, amnesic, muscle relaxant, anticonvulsant | |
| Flubromazolam | Research chemical | ? | ? | hypnotic | |
| Flunitrazepam | Rohypnol, Hipnosedon, Vulbegal, Fluscand, Flunipam, Ronal, Rohydorm, | 1983 | 0.5-3 | 18–26 hours [36–200 hours] (22h=0.92d [118h=4.92d]) | hypnotic |
| Flurazepam | Dalmadorm, Dalmane | 1970 | 1-1.5 | 40–250 hours (145h=6.04d) | hypnotic |
| Flutazolam | Coreminal | ? | 3.5 hours | hypnotic | |
| Flutoprazepam | Restas | 0.5-9 | 60–90 hours (75h=3.12d) | hypnotic, anticonvulsant | |
| Halazepam | Paxipam | 1981 | 1-3 | 30–100 hours (65h=2.71d) | anxiolytic |
| Ketazolam | Anxon | N/A | 2.5-3 | 30–100 hours [36–200] (65h=2.71d [118h=4.92d]) | anxiolytic |
| Loprazolam | Dormonoct | 0.5-4 | 3.3–14.8 hours (9.05h=0.38d) | hypnotic | |
| Lorazepam | Ativan, Lorenin, Lorsilan, Temesta, Tavor, Lorabenz | 1977 | 2-4 | 9.5–20 hours (14.75h=0.61d) | anxiolytic, amnesic, anticonvulsant, hypnotic, muscle relaxant[13][14][15] |
| Lormetazepam | Loramet, Noctamid, Pronoctan | 0.5-2 | 10 hours (=0.42d) | hypnotic | |
| Medazepam | Nobrium, Ansilan, Mezapam, Rudotel, Raporan | 1-1.5 | 36–200 hours (118h=4.92d) | anxiolytic | |
| Mexazolam | Melex | 1-2 | [16] | anxiolytic | |
| Midazolam | Dormicum, Versed, Hypnovel, Dormonid | 1985 | 0.5-1 | 1.5–2.5 hours (2h=0.08d) | hypnotic, anticonvulsant, amnesic, anxiolytic |
| Nifoxipam | Research chemical | ? | ? | hypnotic | |
| Nimetazepam | Erimin | 0.5-3 | 14–30 hours (27h=1.12d) | hypnotic | |
| Nitrazepam | Mogadon, Alodorm, Pacisyn, Dumolid, Nitrazadon | 1965 | 0.5-3 | 17–48 hours (32.5h=1.35d) | hypnotic, anticonvulsant |
| Nordiazepam | Madar, Stilny | ? | 30–150 hours (90h=3.75d) | anxiolytic | |
| Oxazepam | Seresta, Serax, Serenid, Serepax, Sobril, Oxabenz, Oxapax, Opamox | 1965 | 3-4 | 4–11 hours (7.5h=0.31d) | anxiolytic |
| Phenazepam | Phenazepam | 1.5-4 | 60 hours (=2.5d) | anxiolytic, anticonvulsant | |
| Pinazepam | Domar | ? | 40–100 hours (70h=2.92d) | anxiolytic | |
| Prazepam | Lysanxia, Centrax | N/A | 2-6 | 36–200 hours (118h=4.92d) | anxiolytic |
| Premazepam | N/A | 2-6 | 10–13 hours (11.5h=0.48d) | anxiolytic | |
| Pyrazolam | Pyrazolam, Bromazolam | Research chemical | 1-1.5 | 16-18[17] hours (17h=0.71d) | anxiolytic, amnesic |
| Quazepam | Doral | 1985 | 1-5 | 39–120 hours (79.5h=3.31d) | hypnotic |
| Rilmazafone | Rhythmy | ? | [10.5 hours] | hypnotic | |
| Temazepam | Restoril, Normison, Euhypnos, Temaze, Tenox | 1981 | 0.5-3 | 4–10.5 hours (7.25h=0.30d) | hypnotic, anxiolytic, muscle relaxant |
| Thienalprazolam | Thienalprazolam, Deschloroetizolam, Thialprazolam | Research chemical | 1-2 | 10–40 hours (25h=1.04d) | anxiolytic |
| Tetrazepam | Myolastan | 1-3 | 3–26 hours (14.5h=0.60d) | Muscle relaxant | |
| Triazolam | Halcion, Rilamir | 1982 | 0.5-2 | 2 hours (=0.08d) | hypnotic |
Atypical benzodiazepine receptor ligands
| Drug Name | Common Brand Names* | Approval Date (FDA) | Elimination Half-Life (h)† [active metabolite] | Primary Effects |
| DMCM | ? | ? | anxiogenic, convulsant | |
| Flumazenil** | Anexate, Lanexat, Mazicon, Romazicon | 1 hour | antidote | |
| Eszopiclone§ | Lunesta | 2004 | 6 hours | hypnotic |
| Zaleplon§ | Sonata, Starnoc | 1999 | 1 hour | hypnotic |
| Zolpidem§ | Ambien, Nytamel, Sanval, Stilnoct, Stilnox, Sublinox (Canada), Xolnox, Zoldem, Zolnod | 1992 | 2.6 hours | hypnotic |
| Zopiclone§ | Imovane, Rhovane, Ximovan; Zileze; Zimoclone; Zimovane; Zopitan; Zorclone, | 4–6 hours | hypnotic |
* Not all trade names are listed. Click on drug name to see a more comprehensive list.
** Flumazenil is an imidazobenzodiazepine derivative,[18] and in layman's terms, it is a benzodiazepine overdose antidote that is given intravenously in Intensive Care Units (ICUs) to reverse the effects of benzodiazepine overdoses, as well for overdoses of the non-benzodiazepine "Z-drugs" such as Ambien and Lunesta.[19] Flumazenil is contraindicated for benzodiazepine-tolerant patients in overdose cases.[20] In such cases, the benefits are far outweighed by the risks, which include potential and severe seizures.[18][21] The method by which Flumazenil acts to prevent non-benzodiazepine tolerant overdose from causing potential harm is via preventing the benzodiazepines and Z-drugs from binding to the GABAA receptors via competitive inhibition which the Flumazenil creates. Clinical observation notating the patient's oxygen levels, respiratory, heart and blood pressure rates are used, as they are much safer than the potential seizure effects from Flumazenil. Supportive care to mediate any problems resulting from abnormal rates of the pulmonary, respiratory, and cardiovascular systems is typically the only treatment that is required in benzodiazepine-only overdoses.[22] In most cases, activated charcoal/carbon is often used to prevent benzodiazepines from being absorbed by the gastrointestinal tract, and the use of stomach-pumping/gastric lavage is no longer commonly used nor suggested by some toxicologists.[23] Even in cases where other central nervous system (CSN) depressants (such as in combined benzodiazepine and tricyclic antidepressant/TCA overdoses) are detected and/or suspected, endotrachial intubation for the airway path and supportive oxygen are typically implemented and are much safer than Flumazenil.[22]
Controversy
The UK's House of Commons has attempted to get a two to four week limit mandate for prescribing benzodiazepines to replace the two to four week benzodiazepine prescribing guidelines, which are merely recommended.[24][25]
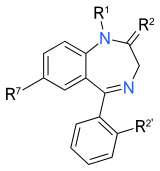
Binding data and structure-activity relationship
A large number of benzodiazepine derivatives have been synthesised and their structure-activity relationships explored in detail.[26][27] This chart contains binding data for benzodiazepines and related drugs investigated by Roche up to the late 1990s (though in some cases the compounds were originally synthesised by other companies such as Takeda or Upjohn).[28][29][30][31][32][33] Other benzodiazepines are also listed for comparison purposes, but it does not however include binding data for;
- Benzodiazepines developed in the former Soviet Union (e.g. phenazepam, gidazepam etc.)
- Benzodiazepines predominantly used only in Japan (e.g. nimetazepam, flutoprazepam etc.)
- 4,5-cyclised benzodiazepines (e.g. ketazolam, cloxazolam etc.), and other compounds not researched by Roche
- Benzodiazepines developed more recently (e.g. remimazolam, QH-ii-066, Ro48-6791 etc.)
- "Designer" benzodiazepines for which in vitro binding data is unavailable (e.g. flubromazolam, pyrazolam etc.)[34]
While binding or activity data is available for most of these compounds also, the assay conditions vary between sources, meaning that in many cases the values are not suitable for a direct comparison. Many older sources used animal measures of activity (i.e. sedation or anticonvulsant activity) but did not measure in vitro binding to benzodiazepine receptors.[35][36] See for instance Table 2 vs Table 11 in the Chem Rev paper, Table 2 lists in vitro pIC50 values matching those below, while Table 11 has pEC50 values derived from in vivo assays in mice, which show the same activity trends but cannot be compared directly, and includes data for compounds such as diclazepam and flubromazepam which are not available in the main data set.
Also note;
- IC50 / pIC50 values represent binding affinity only and do not reflect efficacy or pharmacokinetics, and some compounds listed are GABAA antagonists rather than agonists (e.g. flumazenil).
- Low IC50 or high pIC50 values indicate tighter binding (pIC50 of 8.0 = IC50 of 10nM, pIC50 of 9.0 = IC50 of 1nM, etc.)
- These are non subtype selective IC50 values averaged across all GABAA receptor subtypes, so subtype selective compounds with strong binding at one subtype but weak at others will appear unusually weak due to averaging of binding values (see e.g. CL-218,872)
- Finally, note that the benzodiazepine core is a privileged scaffold, which has been used to derive drugs with diverse activity that is not limited to the GABAA modulatory action of the classical benzodiazepines,[37] such as devazepide and tifluadom, however these have not been included in the list below. 2,3-benzodiazepines such as tofisopam are also not listed, as these act primarily as AMPA receptor modulators, and are inactive at GABAA receptors.
| Table of benzodiazepines: click to | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
See also
References
- ^ Golombok S, Lader M (August 1984). "The psychopharmacological effects of premazepam, diazepam and placebo in healthy human subjects". Br J Clin Pharmacol. 18 (2): 127–33. doi:10.1111/j.1365-2125.1984.tb02444.x. PMC 1463527. PMID 6148956.
- ^ de Visser SJ, van der Post JP, de Waal PP, Cornet F, Cohen AF, van Gerven JM (January 2003). "Biomarkers for the effects of benzodiazepines in healthy volunteers" (PDF). Br J Clin Pharmacol. 55 (1): 39–50. doi:10.1046/j.1365-2125.2002.t01-10-01714.x. PMC 1884188. PMID 12534639.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Benzodiazepine Names". non-benzodiazepines.org.uk. Retrieved 2009-04-05.
- ^ C. Heather Ashton (March 2007). "Benzodiazepine Equivalence Table". benzo.org.uk. Retrieved 2009-04-05.
- ^ Bob, Dr (July 1995). "Benzodiazepine Equivalence Charts". dr-bob.org. Retrieved 2009-04-05.
- ^ Salzman, Carl (15 May 2004). Clinical geriatric psychopharmacology (4th ed.). USA: Lippincott Williams & Wilkins. pp. 450–453. ISBN 978-0-7817-4380-8.
- ^ Delcò F, Tchambaz L, Schlienger R, Drewe J, Krähenbühl S (2005). "Dose adjustment in patients with liver disease". Drug Saf. 28 (6): 529–45. doi:10.2165/00002018-200528060-00005. PMID 15924505.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Riss, J.; Cloyd, J.; Gates, J.; Collins, S. (Aug 2008). "Benzodiazepines in epilepsy: pharmacology and pharmacokinetics". Acta Neurol Scand. 118 (2): 69–86. doi:10.1111/j.1600-0404.2008.01004.x. PMID 18384456.
- ^ Ashton, Dr. Heather (April 2007). "Benzodiazepine Equivalency Table". Retrieved 22 March 2015.
- ^ van Steveninck AL, et al. (1996). "Pharmacokinetic and pharmacodynamic interactions of bretazenil and diazepam with alcohol". British Journal of Clinical Pharmacology. 41 (6): 565–573. doi:10.1046/j.1365-2125.1996.38514.x. PMC 2042631. PMID 8799523.
- ^ Moosmann, Bisel P, Auwärter V. (2014). "Characterization of the designer benzodiazepine diclazepam and preliminary data on its metabolism and pharmacokinetics". The National Center for Biotechnology. 1 (7–8): 1. doi:10.1002/dta.1628. PMID 24604775.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ J Mass Spectrom (2013). "Detection and identification of the designer benzodiazepine flubromazepam and preliminary data on its metabolism and pharmacokinetics". The National Center for Biotechnology. 1 (11): 1150–9. doi:10.1002/jms.3279. PMID 24259203.
- ^ Shah, Dhwani; Borrensen, Dorothy (2011). "Benzodiazepines: A Guide to Safe Prescribing" (PDF). The Carlat Report: Psychiatry.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Farinde, PharmD, PhD, Abimbola. "Benzodiazepine Equivalency Table". Medscape Reference.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Vancouver Hospital Pharmaceutical Sciences. "Comparison of Benzodiazepines".
- ^ Only active metabolites, chlornordiazepam and chloroxazepam, have appreciable effects, with 1.4 and 76 hour half-lives, respectively. See Psychomotor Effects of Mexazolam vs. Placebo in Healthy Volunteers, Clin Drug Invest. 2002;22(10)
- ^ [1]
- ^ a b http://www.gene.com/download/pdf/romazicon_prescribing.pdf
- ^ "Flumazenil Injection, Solution [App Pharmaceuticals, Llc]". Dailymed.nlm.nih.gov. Retrieved 2014-08-15.
- ^ "DailyMed". Dailymed.nlm.nih.gov. Retrieved 2014-08-15.
- ^ Gary R. Fleisher; Stephen Ludwig; Benjamin K. Silverman (2002). Synopsis of pediatric emergency medicine. Lippincott Williams & Wilkins. pp 409. ISBN 978-0-7817-3274-1. Retrieved 3/22/2013.
- ^ a b http://www.inchem.org/documents/pims/pharm/pim181.htm#DivisionTitle:8.1.1.1 Toxicological analyses. Retrieved 3/21/2013.)
- ^ Vale JA, Kulig K; American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. (2004). "Position paper: gastric lavage". J Toxicol Clin Toxicol 42 (7): 933–943. doi:10.1081/CLT-200045006. PMID 15641639
- ^ "November | 2002". Appgita. Retrieved 15 August 2014.
- ^ "APPG for Involuntary Tranquilliser Addiction". benzo.org.uk. Retrieved 21 March 2015.
- ^ Leo H Sternbach. The benzodiazepine story. Journal of Medicinal Chemistry 1979; 22(1):1-7. DOI: 10.1021/jm00187a001
- ^ Hadjipavlou-Litina, D., & Hansch, C. (1994). Quantitative structure-activity relationships of the benzodiazepines. a review and reevaluation. Chemical Reviews, 94(6), 1483-1505. DOI: 10.1021/cr00030a002
- ^ Haefely W, Kyburz E, Gerecke M, Mohler H. Recent advances in the molecular pharmacology of benzodiazepine receptors and in the structure-activity relationships of their agonists and antagonists. Adv. Drug Res. 1985, 14: 165-322.
- ^ Winkler DA, Burden FR, Watkins AJR. Atomistic Topological Indices Applied to Benzodiazepines using Various Regression Methods. Quantitative Structure-Activity Relationships, January 1998; 17(01): 14-19.
- ^ Thakur A, Thakur M, Khadikar P. Topological modeling of benzodiazepine receptor binding. Bioorg Med Chem. 2003 Nov 17;11(23):5203-7. PMID 14604684
- ^ So, S. S., & Karplus, M. (1996). Genetic neural networks for quantitative structure-activity relationships: improvements and application of benzodiazepine affinity for benzodiazepine/GABAA receptors. Journal of Medicinal Chemistry, 39(26), 5246-5256. DOI: 10.1021/jm960536o
- ^ Claus Braestrup and Mogens Nielsen. Benzodiazepine receptors. Biochemical Studies of CNS Receptors. Handbook of Psychopharmacology. (1983) Springer 2013. ISBN 9781468443615
- ^ Zhang et al. Chemical and computer assisted development of an inclusive pharmacophore for the benzodiazepine receptor. Chapter 7, Biological Inhibitors. Volume 2 of Studies in medicinal chemistry. CRC Press, 2004. ISBN 9783718658794
- ^ Moosmann, Bjoern; King, Leslie A.; Auwärter, Volker (2015). "Designer benzodiazepines: A new challenge". World Psychiatry 14 (2): 248–248. doi:10.1002/wps.20236. ISSN 1723-8617
- ^ Blair T, Webb GA. Electronic factors in the structure-activity relationship of some 1,4-benzodiazepin-2-ones. J Med Chem. 1977 Sep;20(9):1206-10. PMID 926122
- ^ Biagi GL, Barbaro AM, Guerra MC, Babbini M, Gaiardi M, Bartoletti M, Borea PA. Rm values and structure-activity relationship of benzodiazepines. J Med Chem. 1980 Feb;23(2):193-201. PMID 7359533
- ^ Spencer J, Rathnam RP, Chowdhry BZ. 1,4-Benzodiazepin-2-ones in medicinal chemistry. Future Med Chem. 2010 Sep;2(9):1441-9. doi: 10.4155/fmc.10.226. PMID 21426139
Further reading
- Gitlow, Stuart (1 October 2006). Substance Use Disorders: A Practical Guide (2nd ed.). USA: Lippincott Williams and Wilkins. p. 110. ISBN 978-0-7817-6998-3.
- Galanter, Marc; Kleber, Herbert D. (1 July 2008). The American Psychiatric Publishing Textbook of Substance Abuse Treatment (4th ed.). United States of America: American Psychiatric Publishing Inc. p. 216. ISBN 978-1-58562-276-4.
{{cite book}}: Cite has empty unknown parameter:|chapterurl=(help)
