MN-18
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
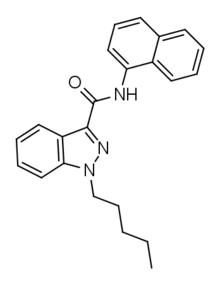
| Formula | C23H23N3O |
| Molar mass | 357.5 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
MN 18 is an indazole-based synthetic cannabinoid that is presumed to be a potent agonist of the CB1 receptor and has been sold online as a designer drug.[1][2] It is the indazole core analogue of NNE1. Given the known metabolic liberation (and presence as an impurity) of amantadine in the related compound APINACA, it is suspected that metabolic hydrolysis of the amide group of MN-18 may release 1-naphthylamine, a known carcinogen.
Legal status
MN-18 is banned in Sweden.[3]
See also
References
- ^ "MN-18". Southern Association of Forensic Scientists. Retrieved 23 July 2015.
- ^ Nahoko Uchiyama; Yoshihiko Shimokawa; Ruri Kikura-Hanajiri; Yosuke Demizu; Yukihiro Goda; Takashi Hakamatsuka (February 2015). "A synthetic cannabinoid FDU-NNEI, two 2H-indazole isomers of synthetic cannabinoids AB-CHMINACA and NNEI indazole analog (MN-18), a phenethylamine derivative N–OH-EDMA, and a cathinone derivative dimethoxy-α-PHP, newly identified in illegal products". Forensic Toxicology. doi:10.1007/s11419-015-0268-7.
- ^ "Cannabinoider föreslås bli klassificerade som hälsofarlig vara". Folkhälsomyndigheten. 28 May 2014. Retrieved 23 July 2015.
