Molar volume
The molar volume, symbol Vm,[1] is the volume occupied by one mole of a substance (chemical element or chemical compound) at a given temperature and pressure. It is equal to the molar mass (M) divided by the mass density (ρ). It has the SI unit cubic metres per mole (m3/mol),[1] although it is more practical to use the units cubic decimetres per mole (dm3/mol) for gases and cubic centimetres per mole (cm3/mol) for liquids and solids.
Calculation
The molar volume of a substance can be found by measuring its molar mass and density then applying the relation
- .
If the sample is a mixture containing N components, the molar volume is complex. It can be simply the sum of the components individualcal components, and calculated using:
- .
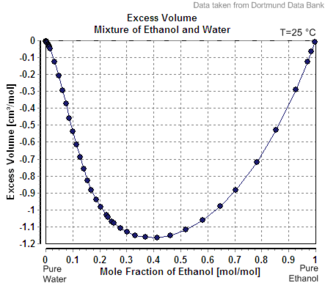
But this is violated by many liquid-liquid mixtures. For instance mixing pure ethanol into pure water causes a decrease in the volume calculated by this formula. This effect is called "excess volume".
Ideal gases
For ideal gases, the molar volume is given by the ideal gas equation: this is a good approximation for many common gases at standard temperature and pressure The ideal gas equation can be rearranged to give an expression for the molar volume of an ideal gas:
- .
Hence, for a given temperature and pressure, the molar volume is the same for all ideal gases and is known to the same precision as the gas constant: R = 0.08206 L atm K−1 mol−1 , that is a relative standard uncertainty of 9.1×10−7, according to the 2010 CODATA recommended value.[2] The molar volume of an ideal gas at 100 kPa (1 bar) is
- 22.710 980(38) (dm)3/mol at 0 °C
- 24.789 598(42) (dm)3/mol at 25 °C
The molar volume of an ideal gas at 1 atmosphere of pressure is
- 22.414 (dm)3/mol at 0 °C
- 24.465 (dm)3/mol at 25 °C
Crystalline solids
For crystalline solids, the molar volume can be measured by X-ray crystallography. The unit cell volume (Vcell) may be calculated from the unit cell parameters, whose determination is the first step in an X-ray crystallography experiment (the calculation is performed automatically by the structure determination software). This is related to the molar volume by
where NA is the Avogadro constant and Z is the number of formula units in the unit cell. The result is normally reported as the "crystallographic density".
Molar volume of silicon
Silicon is routinely made for the electronics industry, and the measurement of the molar volume of silicon, both by X-ray crystallography and by the ratio of molar mass to mass density, has attracted much attention since the pioneering work at NIST by Deslattes et al. (1974).[3] The interest stems from the fact that accurate measurements of the unit cell volume, atomic weight and mass density of a pure crystalline solid provide a direct determination of the Avogadro constant.[4] At present (2006 CODATA recommended value), the precision of the value of the Avogadro constant is limited by the uncertainty in the value of the Planck constant (relative standard uncertainty of 5×10−8).[4][5]
The 2006 CODATA recommended value for the molar volume of silicon is 12.058 8349(11)×10−6 m3/mol, with a relative standard uncertainty of 9.1×10−8.[5]
See also
References
- ^ a b International Union of Pure and Applied Chemistry (1993). Quantities, Units and Symbols in Physical Chemistry, 2nd edition, Oxford: Blackwell Science. ISBN 0-632-03583-8. p. 41. Electronic version.
- ^ "CODATA value: molar gas constant". NIST. Retrieved 2011-10-26.
- ^ Deslattes, R. D.; Henins, A.; Bowman, H. A.; Schoonover, R. M.; Carroll, C. L.; Barnes, I. L.; Machlan, L. A.; Moore, L. J.; Shields, W. R. (1974). "Determination of the Avogadro Constant". Phys. Rev. Lett. 33 (8): 463–66. Bibcode:1974PhRvL..33..463D. doi:10.1103/PhysRevLett.33.463.
- ^ a b Mohr, Peter J.; Taylor, Barry N. (1999). "CODATA recommended values of the fundamental physical constants: 1998" (PDF). Journal of Physical and Chemical Reference Data. 28 (6): 1713–1852. Bibcode:1999JPCRD..28.1713M. doi:10.1063/1.556049. Archived from the original (PDF) on 2017-10-01.
- ^ a b Mohr, Peter J.; Taylor, Barry N.; Newell, David B. (2008). "CODATA Recommended Values of the Fundamental Physical Constants: 2006" (PDF). Reviews of Modern Physics. 80 (2): 633–730. arXiv:0801.0028. Bibcode:2008RvMP...80..633M. doi:10.1103/RevModPhys.80.633. Archived from the original (PDF) on 2017-10-01.
External links
 molar volume (P2807) (see uses)
molar volume (P2807) (see uses)




