Nitrite


The nitrite ion, which has the chemical formula NO2−, is a symmetric anion with equal N-O bond lengths and a O-N-O bond angle of approximately 120°. Upon protonation, the unstable weak acid nitrous acid is produced. Nitrite can be oxidized or reduced, with the product somewhat dependent on the oxidizing/reducing agent and its strength. The nitrite ion is an ambidentate ligand, and is known to bond to metal centers in at least five different ways.[1] Nitrite is also important in biochemistry as a source of the potent vasodilator nitric oxide. In organic chemistry the NO2 group is present in nitrous acid esters and nitro compounds. Nitrites are also used in the food production industry for curing meat.
The nitrite ion
Nitrite salts
Sodium nitrite is made industrially by passing "nitrous fumes" into aqueous sodium hydroxide or sodium carbonate solution:[1]
- NO + NO2 + 2NaOH (or Na2CO3) → 2NaNO2 +H2O ( or CO2)
The product is purified by recrystallization. Alkali metal nitrites are thermally stable up to and beyond their melting point (441 °C for KNO2). Ammonium nitrite can be made from dinitrogen trioxide, N2O3, which is formally the anhydride of nitrous acid:
- 2NH3 + H2O +N2O3 → 2NH4NO2
This compound may decompose explosively on heating.
In organic chemistry nitrites are used in diazotization reactions.
Structure

The nitrite ion has a symmetrical structure (C2v symmetry), with both N-O bonds having equal length. In valence bond theory, it is described as a resonance hybrid with equal contributions from two canonical forms that are mirror images of each other. In molecular orbital theory, there is a sigma bond between each oxygen atom and the nitrogen atom, and a delocalized pi bond made from the p orbitals on nitrogen and oxygen atoms which is perpendicular to the plane of the molecule. The negative charge of the ion is equally distributed on the two oxygen atoms. Both nitrogen and oxygen atoms carry a lone pair of electrons. Therefore, the nitrite ion is a Lewis base. Moreover, it can act as an ambidentate ligand towards a metal ion, donating a pair of electrons from either nitrogen or oxygen atoms.
Acid-base properties
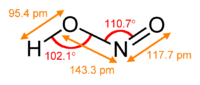
In aqueous solution, nitrous acid is a weak acid:
Nitrous acid is also highly volatile - in the gas phase it exists predominantly as a trans-planar molecule. In solution, it is unstable with respect to the disproportionation reaction:
- 3HNO2 (aq) ⇌ H3O+ + NO3- + 2NO
This reaction is slow at 0 °C.[1] Addition of acid to a solution of a nitrite in the presence of a reducing agent, such as iron (II), is a way to make nitric oxide (NO) in the laboratory.
Oxidation and reduction
The formal oxidation state of the nitrogen atom in a nitrite is +3. This means that it is can be either oxidized to oxidation states +4 and +5, or reduced to oxidation states as low as -3. Standard reduction potentials for reactions directly involving nitrous acid are shown in the table below:[3]
Half-reaction E0/V NO3- + 3H+ + 2e- ⇌ HNO2 + H2O +0.94 2HNO2+ 4H+ + 4e- ⇌ H2N2O2 + 2H2O +0.86 N2O4 + 2H+ + 2e- ⇌ 2HNO2 +1.065 2HNO2+ 4H+ + 4e- ⇌ N2O + 3H2O +1.29
The data can be extended to include products in lower oxidation states. For example:
- H2N2O2 + 2H+ + 2e- ⇌ N2 + 2H2O; E0 = 2.65V
Oxidation reactions usually result in the formation of the nitrate ion, with nitrogen in oxidation state +5. For example, oxidation with permanganate ion can be used for quantitative analysis of nitrite (by titration):
- 5NO2- + 2MnO4- + 6H+ → 5NO3- + 2Mn2+ + 3H2O
The product of reduction reactions with nitrite ion are varied, depending on the reducing agent used and its strength. With sulfur dioxide, the products are NO and N2O; with tin (II), Sn2+, the product is hyponitrous acid, H2N2O2; reduction all the way to ammonia (NH3) occurs with hydrogen sulfide. With the hydrazinium cation, N2H5+, hydrogen azide, HN3, an explosive compound, is produced:
- HNO2 + N2H5+ → HN3 + H2O + H3O+
which can also further react with nitrite:
- HNO2 + HN3 → N2O + N2 + H2O
This reaction is unusual in that it involves compounds with nitrogen in four different oxidation states.[1]
Coordination complexes
The nitrite ion is known to form coordination complexes in at least five different ways.[1]
- When donation is from nitrogen to a metal center, the complex is known as a nitro- complex.
- When donation is from one oxygen to a metal center, the complex is known as a nitrito- complex.
- Both oxygen atoms may donate to a metal center, forming a chelate complex.
- A nitrite ion can form an unsymmetrical bridge between two metal centers, donating through nitrogen to one metal, and through oxygen to the other.
- A single oxygen atom can bridge to two metal centers.
Alfred Werner studied the nitro-nitrito isomerism (1 and 2) extensively. The red isomer of cobalt pentamine with nitrite is now known to be a nitrito complex, [Co(NH3)5(ONO)]2+; it is metastable and isomerizes to the yellow nitro complex [Co(NH3)5(NO2)]2+. An example of chelating nitrite (3) was found in [Cu(bipy)2(O2N)]NO3 - "bipy" is the bidentate ligand 2,2'bypyridyl, with the two bipy ligands occupying four coordination sites on the copper ion, so that the nitrite is forced to occupy two sites in order to achieve an octahedral environment around the copper ion. Examples of 4 and 5 are illustrated.[1]
Nitrite in biochemistry
Sodium nitrite is used for the curing of meat because it prevents bacterial growth and, in a reaction with the meat's myoglobin, gives the product a desirable dark red color. Because of the relatively high toxicity of nitrite (the lethal dose in humans is about 22 milligrams per kilogram of body weight), the maximum allowed nitrite concentration in meat products is 200 ppm. Under certain conditions - especially during cooking - nitrites in meat can react with degradation products of amino acids, forming nitrosamines, which are known carcinogens.[4]
Nitrite is detected and analyzed by the Griess Reaction, involving the formation of a deep red-colored azo dye upon treatment of a NO2−-containing sample with sulfanilic acid and naphthyl-1-amine in the presence of acid.[5] Nitrite can be reduced to nitric oxide or ammonia by many species of bacteria. Under hypoxic conditions, nitrite may release nitric oxide, which causes potent vasodilation. Several mechanisms for nitrite conversion to NO have been described, including enzymatic reduction by xanthine oxidoreductase, nitrite reductase, and NO synthase (NOS), as well as nonenzymatic acidic disproportionation reactions.
Organic nitrites and nitro compounds


In organic chemistry, nitrites are esters of nitrous acid and contain the nitrosoxy functional group. Nitro compounds contain the C-NO2 group. Nitrites have the general formula RONO, where R is an aryl or alkyl group. Amyl nitrite and other alkyl nitrites are used in medicine for the treatment of heart diseases, and occasionally abused recreationally for their "rush" and prolongation of orgasm, particularly in males. A classic named reaction for the synthesis of alkyl nitrites is the Meyer synthesis[6][7] in which alkyl halides react with metallic nitrites to a mixture to nitroalkanes and nitrites.
Nitrobenzene is a simple example of a nitro compound. In aromatic nitration reactions a C-H bond is broken, leaving the two electrons on the carbon atom. These two electrons are added to the nitronium ion, reducing it to nitrite.
See also
References
- ^ a b c d e f Greenwood, pp 461-464
- ^ IUPAC SC-Database A comprehensive database of published data on equilibrium constants of metal complexes and ligands
- ^ Greenwood, p 431
- ^ Template:Cite PMID
- ^ V. M. Ivanov (2004). "The 125th Anniversary of the Griess Reagent". Journal of Analytical Chemistry. 59 (10): 1002–1005. doi:10.1023/B:JANC.0000043920.77446.d7. Translated from V. M. Ivanov (2004). Zhurnal Analiticheskoi Khimii. 59 (10): 1109–1112.
{{cite journal}}: Missing or empty|title=(help) - ^ Victor Meyer (1872). "Ueber die Nitroverbindungen der Fettreihe". Justus Liebig's Annalen der Chemie. 171 (1): 1–56. doi:10.1002/jlac.18741710102.; Victor Meyer, J. Locher (1876). "Ueber die Pseudonitrole, die Isomeren der Nitrolsäuren". Justus Liebig's Annalen der Chemie. 180 (1–2): 133–155. doi:10.1002/jlac.18761800113.; V. Meyer and Stüber (1872). "Vorläufige Mittheilung". Chemische Berichte. 5: 203–205. doi:10.1002/cber.18720050165.; Victor Meyer, O. Stüber (1872). "Ueber die Nitroverbindungen der Fettreihe". Chemische Berichte. 5: 399. doi:10.1002/cber.187200501121.; Victor Meyer, A. Rilliet (1872). "Ueber die Nitroverbindungen der Fettreiche. Dritte Mittheilung". Chemische Berichte. 5 (2): 1029–1034. doi:10.1002/cber.187200502133.; Victor Meyer, C. Chojnacki (1872). "Ueber die Nitroverbindungen der Fettreihe. Vierte Mittheilung". Chemische Berichte. 5 (2): 1034–1038. doi:10.1002/cber.187200502134.
- ^ Robert B. Reynolds, Homer Adkins (1929). "The Relationship of the Constitution of Certain Alky Halides to the Formation of Nitroparaffins and Alkyl Nitrites". Journal of the American Chemical Society. 51 (1): 279–287. doi:10.1021/ja01376a037.
External links
- Material Safety Data Sheet, sodium nitrite
- ATSDR - Case Studies in Environmental Medicine - Nitrate/Nitrite Toxicity U.S. Department of Health and Human Services (public domain)
Bibliography
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
