Nucleotide sugar
Nucleotide sugars are the activated forms of monosaccharides. Nucleotide sugars act as glycosyl donors in glycosylation reactions. Those reactions are catalyzed by a group of enzymes called glycosyltransferases.
History
The anabolism of oligosaccharides - and, hence, the role of nucleotide sugars - was not clear until the 1950s when Leloir and his coworkers found that the key enzymes in this process are the glycosyltransferases. These enzymes transfer a glycosyl group from a sugar nucleotide to an acceptor.[1]
Biological Importance and Energetics
To act as glycosyl donors, those monosaccharides should exist in a highly energetic form. This occurs as a result of a reaction between nucleoside triphosphate (NTP) and glycosyl monophosphate (phosphate at anomeric carbon). The recent discovery of the reversibility of many glycosyltransferase-catalyzed reactions calls into question the designation of sugar nucleotides as 'activated' donors.[2][3][4][5][6]

Types
There are nine sugar nucleotides in complex animals which act as glycosyl donors and they can be classified depending on the type of the nucleoside forming them:[7]
- Uridine Diphosphate: UDP-α-D-Glc, UDP-α-D-Gal, UDP-α-D-GalNAc, UDP-α-D-GlcNAc, UDP-α-D-GlcA, UDP-α-D-Xyl
- Guanine Diphosphate: GDP-α-D-Man, GDP-β-L-Fuc.
- Cytosine Monophosphate: CMP-β-D-Neu5Ac, it is the only nucleotide sugar in the form of nucleotide monophosphate.
In other forms of life many other sugars are used and various donors are utilized for them. All five of the common nucleosides are used as a base for a nucleotide sugar donor somewhere in nature. As examples, CDP-glucose and TDP-glucose give rise to various other forms of CDP and TDP-sugar donor nucleotides.[8][9]
Nucleoside diphosphate glucose
Nucleoside diphosphate glucose, often abbreviated NDP-glucose, refers to nucleotide sugars including glucose.
Structures
listed below are the structures of some nucleotide sugars (example from each type).
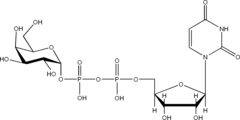
|

|

|
| UDP-Gal | CMP-NeuNAc | GDP-Man |
Relationship to Disease
Normal metabolism of nucleotide sugars is very important. Any malfunction in any contributing enzyme will lead to a certain disease [10] for example:
- Inclusion body myopathy: is a congenital disease resulted from altered function of UDP-GlcNAc epimerase .
- Macular corneal dystrophy: is a congenital disease resulted from malfunction of GlcNAc-6-sulfotransferase.
- Congenital disorder in α-1,3 mannosyl transferase will result in a variety of clinical symptoms, e.g. hypotonia, psychomotor retardation, liver fibrosis and various feeding problems.
Relationship to Drug Discovery
The development of chemoenzymatic strategies to generate large libraries of non-native sugar nucleotides has enabled a process referred to as glycorandomization where these sugar nucleotide libraries serve as donors for permissive glycosyltransferases to afford differential glycosylation of a wide range of pharmaceuticals and complex natural product-based leads.[11][12]
See also
References
- ^ Derek Horton (2008). "The Development of Carbohydrate Chemistry and Biology". Carbohydrate Chemistry, Biology and Medical Applications: 1–28. doi:10.1016/B978-0-08-054816-6.00001-X. ISBN 978-0-08-054816-6.
- ^ Zhang, C; Griffith, BR; Fu, Q; Albermann, C; Fu, X; Lee, IK; Li, L; Thorson, JS (1 September 2006). "Exploiting the reversibility of natural product glycosyltransferase-catalyzed reactions". Science (New York, N.Y.). 313 (5791): 1291–4. doi:10.1126/science.1130028. PMID 16946071.
- ^ Zhang, C; Albermann, C; Fu, X; Thorson, JS (27 December 2006). "The in vitro characterization of the iterative avermectin glycosyltransferase AveBI reveals reaction reversibility and sugar nucleotide flexibility". Journal of the American Chemical Society. 128 (51): 16420–1. doi:10.1021/ja065950k. PMID 17177349.
- ^ Zhang, C; Fu, Q; Albermann, C; Li, L; Thorson, JS (5 March 2007). "The in vitro characterization of the erythronolide mycarosyltransferase EryBV and its utility in macrolide diversification". Chembiochem : a European journal of chemical biology. 8 (4): 385–90. doi:10.1002/cbic.200600509. PMID 17262863.
- ^ Zhang, C; Moretti, R; Jiang, J; Thorson, JS (13 October 2008). "The in vitro characterization of polyene glycosyltransferases AmphDI and NysDI". Chembiochem : a European journal of chemical biology. 9 (15): 2506–14. doi:10.1002/cbic.200800349. PMID 18798210.
- ^ Gantt, RW; Peltier-Pain, P; Cournoyer, WJ; Thorson, JS (21 August 2011). "Using simple donors to drive the equilibria of glycosyltransferase-catalyzed reactions". Nature chemical biology. 7 (10): 685–91. doi:10.1038/nchembio.638. PMID 21857660.
- ^ Cold Spring Harbor Laboratory Press Essentials of Glycobiology, Second Edition
- ^ Samuel G, Reeves P (2003). "Biosynthesis of O-antigens: genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly". Carbohydr. Res. 338 (23): 2503–19. doi:10.1016/j.carres.2003.07.009. PMID 14670712.
- ^ Xue M. He and Hung-wen Liu (2002). "Formation of unusual sugars: Mechanistic studies and biosynthetic applications". Annu Rev Biochem. 71: 701–754. doi:10.1146/annurev.biochem.71.110601.135339. PMID 12045109.
- ^ Encyclopedia of Biological Chemistry, Volume 2. 2004, Elsevier Inc. Hudson H. Freeze 302-307.
- ^ Langenhan, JM; Griffith, BR; Thorson, JS (November 2005). "Neoglycorandomization and chemoenzymatic glycorandomization: two complementary tools for natural product diversification". Journal of natural products. 68 (11): 1696–711. doi:10.1021/np0502084. PMID 16309329.
- ^ Gantt, RW; Peltier-Pain, P; Thorson, JS (October 2011). "Enzymatic methods for glyco(diversification/randomization) of drugs and small molecules". Natural product reports. 28 (11): 1811–53. doi:10.1039/c1np00045d. PMID 21901218.
External links
- "Nucleotide sugars" at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
