Partial androgen insensitivity syndrome
| Partial androgen insensitivity syndrome | |
|---|---|
| Specialty | Endocrinology |
Partial androgen insensitivity syndrome (PAIS) is a condition that results in the partial inability of the cell to respond to androgens.[1][2][3] The partial unresponsiveness of the cell to the presence of androgenic hormones impairs the masculinization of male genitalia in the developing fetus, as well as the development of male secondary sexual characteristics at puberty, but does not significantly impair female genital or sexual development.[3][4] As such, the insensitivity to androgens is only clinically significant when it occurs in genetic males (i.e. individuals with a Y chromosome, or more specifically, an SRY gene).[1]
PAIS is one of three types of androgen insensitivity syndrome, which is divided into three categories that are differentiated by the degree of genital masculinization: complete androgen insensitivity syndrome (CAIS) is indicated when the external genitalia is that of a normal female, mild androgen insensitivity syndrome (MAIS) is indicated when the external genitalia is that of a normal male, and partial androgen insensitivity syndrome (PAIS) is indicated when the external genitalia is partially, but not fully masculinized.[1][2][5][6][7][8][9][10][11]
Androgen insensitivity syndrome is the largest single entity that leads to 46,XY undermasculinization.[12]
Signs and symptoms
A supplemental system of phenotypic grading that uses seven classes instead of the traditional three was proposed by pediatric endocrinologist Charmian A. Quigley et al. in 1995.[3] The first six grades of the scale, grades 1 through 6, are differentiated by the degree of genital masculinization; grade 1 is indicated when the external genitalia is fully masculinized, grade 6 is indicated when the external genitalia is fully feminized, and grades 2 through 5 quantify four degrees of increasingly feminized genitalia that lie in the interim.[3] Grade 7 is indistinguishable from grade 6 until puberty, and is thereafter differentiated by the presence of secondary terminal hair; grade 6 is indicated when secondary terminal hair is present, whereas grade 7 is indicated when it is absent.[3] The Quigley scale can be used in conjunction with the traditional three classes of AIS to provide additional information regarding the degree of genital masculinization, and is particularly useful when the diagnosis is PAIS.[2][13]

Partial androgen insensitivity syndrome is diagnosed when the degree of androgen insensitivity in an individual with a 46,XY karyotype is great enough to partially prevent the masculinization of the genitalia, but is not great enough to completely prevent genital masculinization.[1][2][15][16] This includes any phenotype resulting from androgen insensitivity where the genitalia is partially, but not completely masculinized. Genital ambiguities are frequently detected during clinical examination at birth, and consequently, a PAIS diagnosis can be made during infancy as part of a differential diagnostic workup.[17][18]
Pubertal undervirilization is common, including gynecomastia, decreased secondary terminal hair, and / or a high pitched voice.[19] The phallic structure ranges from a penis with varying degrees of diminished size and hypospadias to a slightly enlarged clitoris.[1][2][3] Wolffian structures (the epididymides, vasa deferentia, and seminal vesicles) are typically partially or fully developed.[2] The prostate is typically small or impalpable.[20][21] Müllerian remnants are rare, but have been reported.[22][23]
The gonads in individuals with PAIS are testes, regardless of phenotype;[2] during the embryonic stage of development, testes form in an androgen-independent process that occurs due to the influence of the SRY gene on the Y chromosome.[24][25] Cryptorchidism is common,[1][2] and carries with it a 50% risk of germ cell malignancy.[26] If the testes are located intrascrotally, there may still be significant risk of germ cell malignancy; studies have not yet been published to assess this risk.[26]
Predominantly male phenotypes vary in the degree of genital undermasculinization to include micropenis, chordee, bifid scrotum, and / or pseudovaginal perineoscrotal hypospadias.[1][15][27] Impotence may be fairly common, depending on phenotypic features; in one study of 15 males with PAIS, 80% of those interviewed indicated that they had some degree of impotence.[28] Anejaculation appears to occur somewhat independently of impotence; some men are still able to ejaculate despite impotence, and others without erectile difficulties cannot.[20][29][30][31] Predominantly female phenotypes include a variable degree of labial fusion and clitoromegaly.[3] Ambiguous phenotypic states include a phallic structure that is intermediate between a clitoris and a penis, and a single perineal orifice that connects to both the urethra and the vagina (i.e. urogenital sinus).[3] At birth, it may not be possible to immediately differentiate the external genitalia of individuals with PAIS as being either male or female,[1][32] although the majority of individuals with PAIS are raised male.[1]
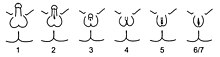
Given the wide diversity of phenotypes associated with PAIS, the diagnosis is often further specified by assessing genital masculinization.[2][3] Grades 2 through 5 of the Quigley scale quantify four degrees of increasingly feminized genitalia that correspond to PAIS.[3]
Grade 2, the mildest form of PAIS, presents with a predominantly male phenotype that presents with minor signs of undermasculinized genitalia, such as isolated hypospadias,[3] which can be severe.[1] Hypospadias may manifest with a partially formed channel from the urethral opening to the glans.[3][33] Until recently, it was thought that isolated micropenis was not a manifestation of PAIS.[1] However, in 2010, two cases of PAIS manifesting with isolated micropenis were documented.[34]

Grade 3, the most common phenotypic form of PAIS,[1][28] features a predominantly male phenotype that is more severely undermasculinized, and typically presents with micropenis and pseudovaginal perineoscrotal hypospadias with bifid scrotum.[3]
Grade 4 presents with a gender ambiguous phenotype, including a phallic structure that is intermediate between a clitoris and a penis.[3] The urethra typically opens into a common channel with the vagina (i.e. urogenital sinus).[3]
Grade 5, the form of PAIS with the greatest degree of androgen insensitivity, presents with a mostly female phenotype, including separate urethral and vaginal orifices, but also shows signs of slight masculinization including mild clitoromegaly and / or partial labial fusion.[1][3]
Previously, it was erroneously thought that individuals with PAIS were always infertile; at least one case report has been published that describes fertile men that fit the criteria for grade 2 PAIS (micropenis, penile hypospadias, and gynecomastia).[36]
Comorbidity

All forms of androgen insensitivity are associated with infertility, though exceptions have been reported for both the mild and partial forms.[4][5][7][36][38][39]
PAIS is associated with a 50% risk of germ cell malignancy when the testes are undescended.[26] If the testes are located intrascrotally, there may still be significant risk of germ cell malignancy; studies have not yet been published to assess this risk.[26] Some men with PAIS may experience sexual dysfunction including impotence and anejaculation.[20][28][29][30][31] A few AR mutations that cause PAIS are also associated with prostate [40][41] and breast [33][42] cancers.
Vaginal hypoplasia, a relatively frequent finding in CAIS and some forms of PAIS,[43][44] is associated with sexual difficulties including vaginal penetration difficulties and dyspareunia.[44][45]
At least one study indicates that individuals with an intersex condition may be more prone to psychological difficulties, due at least in part to parental attitudes and behaviors,[46] and concludes that preventative long-term psychological counseling for parents as well as for affected individuals should be initiated at the time of diagnosis.
Lifespan is not thought to be affected by AIS.[1]
Diagnosis
Unfortunately, the number of differentials to consider for PAIS is particularly large.[1] Prompt diagnosis is particularly urgent when a child is born with ambiguous genitalia, as some causes are associated with potentially life-threatening adrenal crises.[24] Determination of testosterone, testosterone precursors and dihydrotestosterone (DHT) at baseline and / or after human chorionic gonadotropin (hCG) stimulation can be used to exclude such defects in androgen biosynthesis.[2]
Approximately one half of all 46,XY individuals born with ambiguous genitalia will not receive a definitive diagnosis.[47] Androgen receptor (AR) gene mutations cannot be found in 27% [6][29] to 72% [48] of individuals with PAIS. As a result, genetic analysis can be used to confirm a diagnosis of PAIS, but it cannot be used to rule out PAIS.[49] Evidence of abnormal androgen binding in a genital skin fibroblast study has long been the gold standard for the diagnosis of PAIS,[3][50] even when an AR mutation is not present.[47] However, some cases of PAIS, including AR-mutant-positive cases,[30] will show normal androgen binding. A family history consistent with X-linked inheritance is more commonly found in AR-mutant-positive cases than AR-mutant-negative cases.[49]
The use of dynamic endocrine tests is particularly helpful in isolating a diagnosis of PAIS.[1][12] One such test is the human chorionic gonadotropin (hCG) stimulation test. If the gonads are testes, there will be an increase in the level of serum testosterone in response to the hCG, regardless of testicular descent.[1] The magnitude of the testosterone increase can help differentiate between androgen resistance and gonadal dysgenesis, as does evidence of a uterus on ultrasound examination.[1] Testicular function can also be assessed by measuring serum anti-Müllerian hormone levels, which in turn can further differentiate PAIS from gonadal dysgenesis and bilateral anorchia.[1]
Another useful dynamic test involves measuring the response to exogenous steroids; individuals with AIS show a decreased response in serum sex hormone binding globulin (SHBG) after a short term administration of anabolic steroids.[51][52] Two studies [51][52] indicate that measuring the response in SHBG after the administration of stanozolol could help to differentiate individuals with PAIS from those with other causes of ambiguous genitalia, although the response in individuals with predominantly male phenotypes overlaps somewhat with the response in normal males.
Management
Management of AIS is currently limited to symptomatic management; methods to correct a malfunctioning androgen receptor protein that result from an AR gene mutation are not currently available. Areas of management include sex assignment, genitoplasty, gonadectomy in relation to tumor risk, hormone replacement therapy, and genetic and psychological counseling.
Sex assignment
The decision of whether to raise an individual with PAIS as a boy or a girl may not be obvious; grades 3 and 4 in particular present with a phenotype that may be difficult to classify as primarily male or female, and some will be incapable of virilization at puberty.[1][28][32] Parents of an affected newborn should seek immediate help at a center with an experienced multidisciplinary team, and should avoid gender assignment beforehand.[26] Gender assignment should thereafter be expeditiously decided; current guidelines advise against waiting for the child to decide for his / herself.[26] Key considerations involved in assigning gender include the appearance of the genitalia,[26] the extent to which the child can virilize at puberty,[2] surgical options and the postoperative sexual function of the genitalia,[29][43][53] genitoplasty complexity,[26] potential for fertility,[26] and the projected gender identity of the child.[54] The majority of individuals with PAIS are raised male.[1]
Virilization capacity can be assessed by measuring the response to a trial of exogenous androgens; some studies have measured the growth of the phallus in response to exogenous testosterone [32] or dihydrotestosterone,[4] while others have measured the change in sex hormone binding globulin (SHBG) in response to the artificial androgen stanozolol to assess androgen sensitivity.[51][52] Some experts have cautioned that it remains to be proved that a good response to exogenous androgens in neonates is a good predictor of androgen response at puberty.[2] If a mutation in the AR gene is found, it is important to determine if the mutation is inherited or de novo (i.e. a somatic mutation); a certain amount of the wild-type androgen receptor will be present in cases of somatic mutation, which can induce virilization at puberty.[32] A genital skin fibroblast study [3][50] and a human chorionic gonadotropin (hCG) stimulation test [12] may also provide information helpful in the assessment of virilization capacity.
Psychosexual development is influenced by many factors, including the timing, amount, and type of androgen exposure, receptor functionality, and environment, and is thus difficult to predict.[53][54][55][56][57][58] Gender identity begins to develop before 3 years of age,[59] although the earliest age at which it can be reliably assessed has yet to be determined.[26] Approximately 25% of individuals with PAIS are dissatisfied with their assigned gender, regardless of being raised as male or female.[21] One study reports that 46,XY individuals born with micropenis and no hypospadias are better off being raised male, despite the success of some being raised female.[60] Studies involving the more ambiguous phenotypic forms of PAIS are less decisive.[21][28] Homosexuality with respect to assigned gender [15] and atypical gender role behavior [26] are known to occur more frequently in individual with PAIS, and may occur with or without gender dysphoria; neither should be interpreted as an indication of incorrect gender assignment.[26] If an affected child does express feelings of gender dysphoria, the opportunity to explore such feelings with a psychologist experienced in treating intersex conditions should be accommodated.[26] If feelings of gender dysphoria persist, gender reassignment should be initiated, possibly with the aid of a specialist in the field.[26]
Genitoplasty
Genitoplasty, unlike gender assignment, can be irreversible,[49] and there is no guarantee that adult gender identity will develop as assigned despite surgical intervention.[53] Some aspects of genitoplasty are still being debated; a variety of different opinions have been presented by professionals, self-help groups, and patients over the last few decades.[2][61] Points of consideration include what conditions justify genitoplasty, the extent and type of genitoplasty that should be employed, when genitoplasty should be performed, and what the goals of genitoplasty should be.[21][26][53][54][62] Gender assignment itself does not predicate the need for immediate genitoplasty; in some cases, surgical intervention can be delayed to allow the affected child to reach an age and maturity sufficient to have a role in such decisions.[49] Some studies suggest that early surgeries can still produce satisfactory outcomes,[21][63] while others suggest it to be unlikely.[62] Even surgeries that are planned as one-stage procedures often require further major surgery.[62] Scarring and tissue loss that result from repeated surgical procedures are of particular concern, due to the presumed negative impact on sexual function.[21]
While it is thought that feminizing genitoplasty typically requires fewer surgeries to achieve an acceptable result and results in fewer urologic difficulties,[21] there is no evidence that feminizing surgery results in a better psychosocial outcome.[53] In one study,[21] individuals with grade 3 PAIS that were raised male rated their body image and sexual function similarly to those that were raised female, even though they were more likely to have genitalia that was abnormal in size and appearance; more than half of the male participants had a stretched penile length that was below 2.5 standard deviations of the mean, while only 6% of female participants presented with a short vagina in adulthood, and participating physicians gave a lower cosmetic rating to the surgical results of the men than the women. Both male and female participants cited the appearance of their genitalia as being the greatest contributing factor to their dissatisfaction with their body image. In two larger studies,[64][65] the common predictor of gender reassignment was stigmatization related to having an intersex condition.
The outcome of masculinizing genitoplasty is dependent on the amount of erectile tissue and the extent of hypospadias.[26] Procedures include correction of penile curvature and chordee, reconstruction of the urethra, hypospadias correction, orchidopexy, and Müllerian remnant removal to prevent infection and pseudo-incontinence.[1][66] Erectile prosthesis may be inserted in cases of successful neophalloplasty in adulthood, although it has a high morbidity.[26] Additional surgeries may be required to correct postsurgical complications such as stenosis of the anastomosis between the native urethra and the graft, urethral fistulas, and posterior displacement of the balanic meatus.[66] Successful masculinizing genitoplasty performed on individuals with grade 3 PAIS often requires multiple surgeries.[21]

If feminizing genitoplasty is performed in infancy, the result will need to be refined at puberty through additional surgery.[68] Procedures include clitoral reduction / recession, labiaplasty, repair of the common urogenital sinus, vaginoplasty, and vaginal dilation through non-surgical pressure methods.[26][44][53][68] Clitoral reduction / recession surgery carries with it the risk of necrosis [68] as well as the risk of impairing the sexual function of the genitalia,[53] and thus should not be performed for less severe clitoromegaly.[26] Clitoral surgery should be focused on function rather than appearance, with care being taken to spare the erectile function and innervation of the clitoris.[26] If PAIS presents with a common urogenital sinus, the American Academy of Pediatrics currently recommends that surgery to separate the urethra from the vagina be performed at an early age.[69] As is the case for CAIS, vaginal dilation using pressure dilation methods should be attempted before the surgical creation of a neovagina is considered, and neither should be performed before puberty.[26][44] Complications of feminizing genitoplasty can include vaginal stenosis, meatal stenosis, vaginourethral fistula, female hypospadias, urinary tract injuries, and recurrent clitoromegaly.[44][67] Successful feminizing genitoplasty performed on individuals with grade 3 PAIS often requires multiple surgeries, although more surgeries are typically required for successful masculinizing genitoplasty in this population.[21]
Many surgical procedures have been developed to create a neovagina, as none of them is ideal.[44] Surgical intervention should only be considered after non-surgical pressure dilation methods have failed to produce a satisfactory result.[44] Neovaginoplasty can be performed using skin grafts, a segment of bowel, ileum, peritoneum, Interceed,[70][71] buccal mucosa, amnion, or dura mater.[44][67][72] Success of such methods should be determined by sexual function, and not just by vaginal length, as has been done in the past.[67] Ileal or cecal segments may be problematic because of a shorter mesentery, which may produce tension on the neovagina, leading to stenosis.[67] The sigmoid neovagina is thought to be self-lubricating, without the excess mucus production associated with segments of small bowel.[67] Vaginoplasty may create scarring at the introitus (the vaginal opening), which requires additional surgery to correct. Vaginal dilators are required postoperatively to prevent vaginal stenosis from scarring.[43][44] Other complications include bladder and bowel injuries.[44] Yearly exams are required as neovaginoplasty carries a risk of carcinoma,[44] although carcinoma of the neovagina is uncommon.[67][72] Neither neovaginoplasty nor vaginal dilation should be performed before puberty.[26][44]
Gonadectomy
Gonadectomy at time of diagnosis is the current recommendation for PAIS if presenting with cryptorchidism, due to the high (50%) risk of germ cell malignancy.[26] The risk of malignancy when testes are located intrascrotally is unknown; the current recommendation is to biopsy the testes at puberty, allowing investigation of at least 30 seminiferous tubules, with diagnosis preferably based on OCT3/4 immunohistochemistry, followed by regular examinations.[26] Hormone replacement therapy is required after gonadectomy, and should be modulated over time to replicate the hormone levels naturally present in the body during the various stages of puberty.[26] Artificially induced puberty results in the same, normal development of secondary sexual characteristics, growth spurt, and bone mineral accumulation.[26] Women with PAIS may have a tendency towards bone mineralization deficiency, although this increase is thought to be less than is typically seen in CAIS, and is similarly managed.[73]
Hormonal replacement therapy
Testosterone has been used to successfully treat undervirilization in some [14] but not all [74] men with PAIS, despite having supraphysiological levels of testosterone to start with.[14][75] Treatment options include transdermal gels or patches, oral or injectable testosterone undecanoate, other injectable testosterone esters, testosterone pellets, or buccal testosterone systems.[76] Supraphysiological doses may be required to achieve the desired physiological effect,[14][26][77] which may be difficult to achieve using non-injectable testosterone preparations. Exogenous testosterone supplementation in unaffected men can produce various unwanted side effects, including prostatic hypertrophy, polycythemia, gynecomastia, hair loss, acne, and the suppression of the hypothalamic-pituitary-gonadal axis, which results in the reduction of gonadotropins (i.e., luteinizing hormone and follicle-stimulating hormone) and spermatogenic defect.[78][79] These effects may not manifest at all in men with AIS, or might only manifest at a much higher concentration of testosterone, depending on the degree of androgen insensitivity.[14][74][75] Those undergoing high dose androgen therapy should be monitored for safety and efficacy of treatment, possibly including regular breast [14] and prostate [78] examinations. Some individuals with PAIS have a sufficiently high sperm count to father children; at least one case report has been published that describes fertile men that fit the criteria for grade 2 PAIS (micropenis, penile hypospadias, and gynecomastia).[36] Several publications have indicated that testosterone treatment can correct low sperm counts in men with MAIS.[1][77] At least one case report has been published that documents the efficacy of treating a low sperm count with tamoxifen in an individual with PAIS.[80]
Counseling
Depending on phenotypic features, impotence and other sexual problems such as anejaculation or sexual aversion may be fairly common among individuals with PAIS,[20][28][29][30][31] but do not necessarily indicate low libido.[26][28] Support groups for individuals with PAIS may help affected individuals discuss their concerns more comfortably.[26] Some individuals with PAIS may try to avoid intimate relationships out of fear of rejection; individual therapy may help some to overcome social anxiety, and restore focus to interpersonal relationships instead of solely on sexual function and activity.[26]
References
- ^ a b c d e f g h i j k l m n o p q r s t u v w Hughes IA, Deeb A (December 2006). "Androgen resistance". Best Pract. Res. Clin. Endocrinol. Metab. 20 (4): 577–98. doi:10.1016/j.beem.2006.11.003. PMID 17161333.
- ^ a b c d e f g h i j k l m Galani A, Kitsiou-Tzeli S, Sofokleous C, Kanavakis E, Kalpini-Mavrou A (2008). "Androgen insensitivity syndrome: clinical features and molecular defects". Hormones (Athens). 7 (3): 217–29. doi:10.14310/horm.2002.1201. PMID 18694860.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j k l m n o p q r s Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS (June 1995). "Androgen receptor defects: historical, clinical, and molecular perspectives". Endocr. Rev. 16 (3): 271–321. doi:10.1210/edrv-16-3-271. PMID 7671849.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Giwercman YL, Nordenskjöld A, Ritzén EM, Nilsson KO, Ivarsson SA, Grandell U, Wedell A (June 2002). "An androgen receptor gene mutation (E653K) in a family with congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency as well as in partial androgen insensitivity". J. Clin. Endocrinol. Metab. 87 (6): 2623–8. doi:10.1210/jc.87.6.2623. PMID 12050225.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Zuccarello D, Ferlin A, Vinanzi C, Prana E, Garolla A, Callewaert L, Claessens F, Brinkmann AO, Foresta C (April 2008). "Detailed functional studies on androgen receptor mild mutations demonstrate their association with male infertility". Clin. Endocrinol. (Oxf). 68 (4): 580–8. doi:10.1111/j.1365-2265.2007.03069.x. PMID 17970778.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Ferlin A, Vinanzi C, Garolla A, Selice R, Zuccarello D, Cazzadore C, Foresta C (November 2006). "Male infertility and androgen receptor gene mutations: clinical features and identification of seven novel mutations". Clin. Endocrinol. (Oxf). 65 (5): 606–10. doi:10.1111/j.1365-2265.2006.02635.x. PMID 17054461.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Stouffs K, Tournaye H, Liebaers I, Lissens W (2009). "Male infertility and the involvement of the X chromosome". Hum. Reprod. Update. 15 (6): 623–37. doi:10.1093/humupd/dmp023. PMID 19515807.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ozülker T, Ozpaçaci T, Ozülker F, Ozekici U, Bilgiç R, Mert M (January 2010). "Incidental detection of Sertoli-Leydig cell tumor by FDG PET/CT imaging in a patient with androgen insensitivity syndrome". Ann Nucl Med. 24 (1): 35–9. doi:10.1007/s12149-009-0321-x. PMID 19957213.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Davis-Dao CA, Tuazon ED, Sokol RZ, Cortessis VK (November 2007). "Male infertility and variation in CAG repeat length in the androgen receptor gene: a meta-analysis". J. Clin. Endocrinol. Metab. 92 (11): 4319–26. doi:10.1210/jc.2007-1110. PMID 17684052.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kawate H, Wu Y, Ohnaka K, Tao RH, Nakamura K, Okabe T, Yanase T, Nawata H, Takayanagi R (November 2005). "Impaired nuclear translocation, nuclear matrix targeting, and intranuclear mobility of mutant androgen receptors carrying amino acid substitutions in the deoxyribonucleic acid-binding domain derived from androgen insensitivity syndrome patients". J. Clin. Endocrinol. Metab. 90 (11): 6162–9. doi:10.1210/jc.2005-0179. PMID 16118342.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gottlieb B, Lombroso R, Beitel LK, Trifiro MA (January 2005). "Molecular pathology of the androgen receptor in male (in)fertility". Reprod. Biomed. Online. 10 (1): 42–8. doi:10.1016/S1472-6483(10)60802-4. PMID 15705293.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Ahmed SF, Cheng A, Hughes IA (April 1999). "Assessment of the gonadotrophin-gonadal axis in androgen insensitivity syndrome". Arch. Dis. Child. 80 (4): 324–9. doi:10.1136/adc.80.4.324. PMC 1717906. PMID 10086936.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sultan C, Paris F, Terouanne B, Balaguer P, Georget V, Poujol N, Jeandel C, Lumbroso S, Nicolas JC (2001). "Disorders linked to insufficient androgen action in male children". Hum. Reprod. Update. 7 (3): 314–22. doi:10.1093/humupd/7.3.314. PMID 11392378.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f Weidemann W, Peters B, Romalo G, Spindler KD, Schweikert HU (April 1998). "Response to androgen treatment in a patient with partial androgen insensitivity and a mutation in the deoxyribonucleic acid-binding domain of the androgen receptor". J. Clin. Endocrinol. Metab. 83 (4): 1173–6. doi:10.1210/jc.83.4.1173. PMID 9543136.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Oakes MB, Eyvazzadeh AD, Quint E, Smith YR (December 2008). "Complete androgen insensitivity syndrome--a review". J Pediatr Adolesc Gynecol. 21 (6): 305–10. doi:10.1016/j.jpag.2007.09.006. PMID 19064222.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Decaestecker K, Philibert P, De Baere E, Hoebeke P, Kaufman JM, Sultan C, T'Sjoen G (May 2008). "A novel mutation c.118delA in exon 1 of the androgen receptor gene resulting in complete androgen insensitivity syndrome within a large family". Fertil. Steril. 89 (5): 1260.e3–7. doi:10.1016/j.fertnstert.2007.04.057. PMID 17714709.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lee PA, Brown TR, LaTorre HA (April 1986). "Diagnosis of the partial androgen insensitivity syndrome during infancy". JAMA. 255 (16): 2207–9. doi:10.1001/jama.255.16.2207. PMID 3959303.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bhagabath B, Bradshaw KD (2008). "Non-surgical management of Müllerian anomalies". In Emre S, Aydin A (ed.). Non-Invasive Management of Gynecologic Disorders. Informa Healthcare. pp. 193–202. ISBN 0-415-41742-2.
- ^ Shkolny DL, Beitel LK, Ginsberg J, Pekeles G, Arbour L, Pinsky L, Trifiro MA (February 1999). "Discordant measures of androgen-binding kinetics in two mutant androgen receptors causing mild or partial androgen insensitivity, respectively". J. Clin. Endocrinol. Metab. 84 (2): 805–10. doi:10.1210/jc.84.2.805. PMID 10022458.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Boehmer AL, Brinkmann O, Brüggenwirth H, van Assendelft C, Otten BJ, Verleun-Mooijman MC, Niermeijer MF, Brunner HG, Rouwé CW, Waelkens JJ, Oostdijk W, Kleijer WJ, van der Kwast TH, de Vroede MA, Drop SL (September 2001). "Genotype versus phenotype in families with androgen insensitivity syndrome". J. Clin. Endocrinol. Metab. 86 (9): 4151–60. doi:10.1210/jc.86.9.4151. PMID 11549642.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j Migeon CJ, Wisniewski AB, Gearhart JP, Meyer-Bahlburg HF, Rock JA, Brown TR, Casella SJ, Maret A, Ngai KM, Money J, Berkovitz GD (September 2002). "Ambiguous genitalia with perineoscrotal hypospadias in 46,XY individuals: long-term medical, surgical, and psychosexual outcome". Pediatrics. 110 (3): e31. doi:10.1542/peds.110.3.e31. PMID 12205281.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tanaka Y, Matsuo N, Aya M, et al. (1995). "Persistent Müllerian duct remnants in three siblings with partial androgen insensitivity". Horumon To Rinsho. 43: 3–8.
- ^ Mazur T (August 2005). "Gender dysphoria and gender change in androgen insensitivity or micropenis". Arch Sex Behav. 34 (4): 411–21. doi:10.1007/s10508-005-4341-x. PMID 16010464.
- ^ a b Achermann JC, Jameson JL (2006). "Disorders of sexual differentiation". In Hauser SL, Kasper DL, Fauci AS, Braunwald E, Longo DL (ed.). Harrison's endocrinology. New York: McGraw-Hill Medical Pub. Division. pp. 161–172. ISBN 0-07-145744-5.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ Simpson JL, Rebar RW (2002). Hung, Wellington; Becker, Kenneth L.; Bilezikian, John P.; William J Bremner (ed.). Principles and Practice of Endocrinology and Metabolism. Hagerstwon, MD: Lippincott Williams & Wilkins. pp. 852–885. ISBN 0-7817-4245-5.
{{cite book}}: CS1 maint: multiple names: editors list (link) - ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad Hughes IA, Houk C, Ahmed SF, Lee PA (July 2006). "Consensus statement on management of intersex disorders". Arch. Dis. Child. 91 (7): 554–63. doi:10.1136/adc.2006.098319. PMC 2082839. PMID 16624884.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Evans BA, Hughes IA, Bevan CL, Patterson MN, Gregory JW (June 1997). "Phenotypic diversity in siblings with partial androgen insensitivity syndrome". Arch. Dis. Child. 76 (6): 529–31. doi:10.1136/adc.76.6.529. PMC 1717223. PMID 9245853.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g Bouvattier C, Mignot B, Lefèvre H, Morel Y, Bougnères P (September 2006). "Impaired sexual activity in male adults with partial androgen insensitivity". J. Clin. Endocrinol. Metab. 91 (9): 3310–5. doi:10.1210/jc.2006-0218. PMID 16757528.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e Melo KF, Mendonca BB, Billerbeck AE, Costa EM, Inácio M, Silva FA, Leal AM, Latronico AC, Arnhold IJ (July 2003). "Clinical, hormonal, behavioral, and genetic characteristics of androgen insensitivity syndrome in a Brazilian cohort: five novel mutations in the androgen receptor gene". J. Clin. Endocrinol. Metab. 88 (7): 3241–50. doi:10.1210/jc.2002-021658. PMID 12843171.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Deeb A, Jääskeläinen J, Dattani M, Whitaker HC, Costigan C, Hughes IA (October 2008). "A novel mutation in the human androgen receptor suggests a regulatory role for the hinge region in amino-terminal and carboxy-terminal interactions". J. Clin. Endocrinol. Metab. 93 (10): 3691–6. doi:10.1210/jc.2008-0737. PMID 18697867.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Miller MA, Grant DB (September 1997). "Severe hypospadias with genital ambiguity: adult outcome after staged hypospadias repair". Br J Urol. 80 (3): 485–8. doi:10.1046/j.1464-410x.1997.00348.x. PMID 9313674.
- ^ a b c d Köhler B, Lumbroso S, Leger J, Audran F, Grau ES, Kurtz F, Pinto G, Salerno M, Semitcheva T, Czernichow P, Sultan C (January 2005). "Androgen insensitivity syndrome: somatic mosaicism of the androgen receptor in seven families and consequences for sex assignment and genetic counseling". J. Clin. Endocrinol. Metab. 90 (1): 106–11. doi:10.1210/jc.2004-0462. PMID 15522944.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Wooster R, Mangion J, Eeles R, Smith S, Dowsett M, Averill D, Barrett-Lee P, Easton DF, Ponder BA, Stratton MR (October 1992). "A germline mutation in the androgen receptor gene in two brothers with breast cancer and Reifenstein syndrome". Nat. Genet. 2 (2): 132–4. doi:10.1038/ng1092-132. PMID 1303262.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bhangoo A, Paris F, Philibert P, Audran F, Ten S, Sultan C (July 2010). "Isolated micropenis reveals partial androgen insensitivity syndrome confirmed by molecular analysis". Asian J. Androl. 12 (4): 561–6. doi:10.1038/aja.2010.6. PMID 20305676.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Aguilar-Ponce J, Chilaca Rosas F, Molina Calzada C, Granados García M, Jiménez Ríos MA, De la Garza Salazar J (December 2008). "Testicular cancer in androgen insensitivity syndrome in a Mexican population". Clin Transl Oncol. 10 (12): 840–3. doi:10.1007/s12094-008-0298-2. PMID 19068456.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Chu J, Zhang R, Zhao Z, Zou W, Han Y, Qi Q, Zhang H, Wang JC, Tao S, Liu X, Luo Z (January 2002). "Male fertility is compatible with an Arg(840)Cys substitution in the AR in a large Chinese family affected with divergent phenotypes of AR insensitivity syndrome". J. Clin. Endocrinol. Metab. 87 (1): 347–51. doi:10.1210/jc.87.1.347. PMID 11788673.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Nichols JL, Bieber EJ, Gell JS. Case of sisters with complete androgen insensitivity syndrome and discordant Müllerian remnants. Fertil Steril. 2009;91:932e15-e18.
- ^ Menakaya UA, Aligbe J, Iribhogbe P, Agoreyo F, Okonofua FE (May 2005). "Complete androgen insensitivity syndrome with persistent Mullerian derivatives: a case report". J Obstet Gynaecol. 25 (4): 403–5. doi:10.1080/01443610500143226. PMID 16091340.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Giwercman A, Kledal T, Schwartz M, Giwercman YL, Leffers H, Zazzi H, Wedell A, Skakkebaek NE (June 2000). "Preserved male fertility despite decreased androgen sensitivity caused by a mutation in the ligand-binding domain of the androgen receptor gene". J. Clin. Endocrinol. Metab. 85 (6): 2253–9. doi:10.1210/jc.85.6.2253. PMID 10852459.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lund A, Juvonen V, Lähdetie J, Aittomäki K, Tapanainen JS, Savontaus ML (June 2003). "A novel sequence variation in the transactivation regulating domain of the androgen receptor in two infertile Finnish men". Fertil. Steril. 79 Suppl 3: 1647–8. doi:10.1016/s0015-0282(03)00256-5. PMID 12801573.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Evans BA, Harper ME, Daniells CE, Watts CE, Matenhelia S, Green J, Griffiths K (March 1996). "Low incidence of androgen receptor gene mutations in human prostatic tumors using single strand conformation polymorphism analysis". Prostate. 28 (3): 162–71. doi:10.1002/(SICI)1097-0045(199603)28:3<162::AID-PROS3>3.0.CO;2-H. PMID 8628719.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lobaccaro JM, Lumbroso S, Belon C, Galtier-Dereure F, Bringer J, Lesimple T, Namer M, Cutuli BF, Pujol H, Sultan C (November 1993). "Androgen receptor gene mutation in male breast cancer". Hum. Mol. Genet. 2 (11): 1799–802. doi:10.1093/hmg/2.11.1799. PMID 8281139.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Ismail-Pratt IS, Bikoo M, Liao LM, Conway GS, Creighton SM (July 2007). "Normalization of the vagina by dilator treatment alone in Complete Androgen Insensitivity Syndrome and Mayer-Rokitansky-Kuster-Hauser Syndrome". Hum. Reprod. 22 (7): 2020–4. doi:10.1093/humrep/dem074. PMID 17449508.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j k l Quint EH, McCarthy JD, Smith YR (March 2010). "Vaginal surgery for congenital anomalies". Clin Obstet Gynecol. 53 (1): 115–24. doi:10.1097/GRF.0b013e3181cd4128. PMID 20142648.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Minto CL, Liao KL, Conway GS, Creighton SM (July 2003). "Sexual function in women with complete androgen insensitivity syndrome". Fertil. Steril. 80 (1): 157–64. doi:10.1016/S0015-0282(03)00501-6. PMID 12849818.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Slijper FM, Drop SL, Molenaar JC, de Muinck Keizer-Schrama SM (April 1998). "Long-term psychological evaluation of intersex children". Arch Sex Behav. 27 (2): 125–44. doi:10.1023/A:1018670129611. PMID 9562897.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Morel Y, Rey R, Teinturier C, Nicolino M, Michel-Calemard L, Mowszowicz I, Jaubert F, Fellous M, Chaussain JL, Chatelain P, David M, Nihoul-Fékété C, Forest MG, Josso N (January 2002). "Aetiological diagnosis of male sex ambiguity: a collaborative study". Eur. J. Pediatr. 161 (1): 49–59. doi:10.1007/s00431-001-0854-z. PMID 11808880.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ahmed SF, Cheng A, Dovey L, Hawkins JR, Martin H, Rowland J, Shimura N, Tait AD, Hughes IA (February 2000). "Phenotypic features, androgen receptor binding, and mutational analysis in 278 clinical cases reported as androgen insensitivity syndrome". J. Clin. Endocrinol. Metab. 85 (2): 658–65. doi:10.1210/jc.85.2.658. PMID 10690872.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Hughes IA (February 2008). "Disorders of sex development: a new definition and classification". Best Pract. Res. Clin. Endocrinol. Metab. 22 (1): 119–34. doi:10.1016/j.beem.2007.11.001. PMID 18279784.
- ^ a b Weidemann W, Linck B, Haupt H, Mentrup B, Romalo G, Stockklauser K, Brinkmann AO, Schweikert HU, Spindler KD (December 1996). "Clinical and biochemical investigations and molecular analysis of subjects with mutations in the androgen receptor gene". Clin. Endocrinol. (Oxf). 45 (6): 733–9. doi:10.1046/j.1365-2265.1996.8600869.x. PMID 9039340.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Sinnecker GH, Hiort O, Nitsche EM, Holterhus PM, Kruse K (January 1997). "Functional assessment and clinical classification of androgen sensitivity in patients with mutations of the androgen receptor gene. German Collaborative Intersex Study Group". Eur. J. Pediatr. 156 (1): 7–14. doi:10.1007/s004310050542. PMID 9007482.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Sinnecker G, Köhler S (June 1989). "Sex hormone-binding globulin response to the anabolic steroid stanozolol: evidence for its suitability as a biological androgen sensitivity test". J. Clin. Endocrinol. Metab. 68 (6): 1195–200. doi:10.1210/jcem-68-6-1195. PMID 2723028.
- ^ a b c d e f g Minto CL, Liao LM, Woodhouse CR, Ransley PG, Creighton SM (April 2003). "The effect of clitoral surgery on sexual outcome in individuals who have intersex conditions with ambiguous genitalia: a cross-sectional study". Lancet. 361 (9365): 1252–7. doi:10.1016/S0140-6736(03)12980-7. PMID 12699952.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Meyer-Bahlburg HF (October 1999). "Gender assignment and reassignment in 46,XY pseudohermaphroditism and related conditions". J. Clin. Endocrinol. Metab. 84 (10): 3455–8. doi:10.1210/jc.84.10.3455. PMID 10522979.
- ^ Goy RW, Bercovitch FB, McBrair MC (December 1988). "Behavioral masculinization is independent of genital masculinization in prenatally androgenized female rhesus macaques". Horm Behav. 22 (4): 552–71. doi:10.1016/0018-506X(88)90058-X. PMID 3235069.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wallen K (April 2005). "Hormonal influences on sexually differentiated behavior in nonhuman primates". Front Neuroendocrinol. 26 (1): 7–26. doi:10.1016/j.yfrne.2005.02.001. PMID 15862182.
- ^ Moore CL (1992). "The role of maternal stimulation in the development of sexual behavior and its neural basis". Annals of the New York Academy of Sciences. 662: 160–77. doi:10.1111/j.1749-6632.1992.tb22859.x. PMID 1456637.
- ^ Wallen K (December 1996). "Nature needs nurture: the interaction of hormonal and social influences on the development of behavioral sex differences in rhesus monkeys". Horm Behav. 30 (4): 364–78. doi:10.1006/hbeh.1996.0042. PMID 9047263.
- ^ Martin CL, Ruble DN, Szkrybalo J (November 2002). "Cognitive theories of early gender development". Psychol Bull. 128 (6): 903–33. doi:10.1037/0033-2909.128.6.903. PMID 12405137.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wisniewski AB, Migeon CJ, Gearhart JP, Rock JA, Berkovitz GD, Plotnick LP, Meyer-Bahlburg HF, Money J (2001). "Congenital micropenis: long-term medical, surgical and psychosexual follow-up of individuals raised male or female". Horm. Res. 56 (1–2): 3–11. doi:10.1159/000048083. PMID 11815721.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Zucker KJ (February 2002). "Intersexuality and gender identity differentiation". J Pediatr Adolesc Gynecol. 15 (1): 3–13. doi:10.1016/S1083-3188(01)00133-4. PMID 11888804.
- ^ a b c Creighton SM, Minto CL, Steele SJ (July 2001). "Objective cosmetic and anatomical outcomes at adolescence of feminising surgery for ambiguous genitalia done in childhood". Lancet. 358 (9276): 124–5. doi:10.1016/S0140-6736(01)05343-0. PMID 11463417.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Warne G, Grover S, Hutson J, Sinclair A, Metcalfe S, Northam E, Freeman J (June 2005). "A long-term outcome study of intersex conditions". J. Pediatr. Endocrinol. Metab. 18 (6): 555–67. doi:10.1515/jpem.2005.18.6.555. PMID 16042323.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Money J, Devore H, Norman BF (1986). "Gender identity and gender transposition: longitudinal outcome study of 32 male hermaphrodites assigned as girls". J Sex Marital Ther. 12 (3): 165–81. doi:10.1080/00926238608415404. PMID 3761370.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Money J, Norman BF (1987). "Gender identity and gender transposition: longitudinal outcome study of 24 male hermaphrodites assigned as boys". J Sex Marital Ther. 13 (2): 75–92. doi:10.1080/00926238708403881. PMID 3612827.
- ^ a b Nihoul-Fékété C, Thibaud E, Lortat-Jacob S, Josso N (May 2006). "Long-term surgical results and patient satisfaction with male pseudohermaphroditism or true hermaphroditism: a cohort of 63 patients". J. Urol. 175 (5): 1878–84. doi:10.1016/S0022-5347(05)00934-1. PMID 16600787.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g Breech LL (2008). "Complications of vaginoplasty and clitoroplasty". In Teich S, Caniano DA (ed.). Reoperative pediatric surgery. Totowa, N.J: Humana. pp. 499–514. ISBN 1-58829-761-6.
- ^ a b c Alizai NK, Thomas DF, Lilford RJ, Batchelor AG, Johnson N (May 1999). "Feminizing genitoplasty for congenital adrenal hyperplasia: what happens at puberty?". J. Urol. 161 (5): 1588–91. doi:10.1016/S0022-5347(05)68986-0. PMID 10210421.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ American Academy of Pediatrics (April 1996). "Timing of elective surgery on the genitalia of male children with particular reference to the risks, benefits, and psychological effects of surgery and anesthesia". Pediatrics. 97 (4): 590–4. PMID 8632952.
- ^ Motoyama S, Laoag-Fernandez JB, Mochizuki S, Yamabe S, Maruo T (May 2003). "Vaginoplasty with Interceed absorbable adhesion barrier for complete squamous epithelialization in vaginal agenesis". Am. J. Obstet. Gynecol. 188 (5): 1260–4. doi:10.1067/mob.2003.317. PMID 12748495.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jackson ND, Rosenblatt PL (December 1994). "Use of Interceed Absorbable Adhesion Barrier for vaginoplasty". Obstet Gynecol. 84 (6): 1048–50. PMID 7970464.
- ^ a b Steiner E, Woernle F, Kuhn W, Beckmann K, Schmidt M, Pilch H, Knapstein PG (January 2002). "Carcinoma of the neovagina: case report and review of the literature". Gynecol. Oncol. 84 (1): 171–5. doi:10.1006/gyno.2001.6417. PMID 11748997.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Danilovic DL, Correa PH, Costa EM, Melo KF, Mendonca BB, Arnhold IJ (March 2007). "Height and bone mineral density in androgen insensitivity syndrome with mutations in the androgen receptor gene". Osteoporos Int. 18 (3): 369–74. doi:10.1007/s00198-006-0243-6. PMID 17077943.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Tincello DG, Saunders PT, Hodgins MB, Simpson NB, Edwards CR, Hargreaves TB, Wu FC (April 1997). "Correlation of clinical, endocrine and molecular abnormalities with in vivo responses to high-dose testosterone in patients with partial androgen insensitivity syndrome". Clin. Endocrinol. (Oxf). 46 (4): 497–506. doi:10.1046/j.1365-2265.1997.1140927.x. PMID 9196614.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Pinsky L, Kaufman M, Killinger DW (January 1989). "Impaired spermatogenesis is not an obligate expression of receptor-defective androgen resistance". Am. J. Med. Genet. 32 (1): 100–4. doi:10.1002/ajmg.1320320121. PMID 2705470.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Leichtnam ML, Rolland H, Wüthrich P, Guy RH (June 2006). "Testosterone hormone replacement therapy: state-of-the-art and emerging technologies". Pharm. Res. 23 (6): 1117–32. doi:10.1007/s11095-006-0072-5. PMID 16755346.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Yong EL, Ng SC, Roy AC, Yun G, Ratnam SS (September 1994). "Pregnancy after hormonal correction of severe spermatogenic defect due to mutation in androgen receptor gene". Lancet. 344 (8925): 826–7. doi:10.1016/S0140-6736(94)92385-X. PMID 7993455.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Nieschlag E (September 2006). "Testosterone treatment comes of age: new options for hypogonadal men". Clin. Endocrinol. (Oxf). 65 (3): 275–81. doi:10.1111/j.1365-2265.2006.02618.x. PMID 16918944.
- ^ Handelsman DJ, Conway AJ, Boylan LM (November 1992). "Suppression of human spermatogenesis by testosterone implants". J. Clin. Endocrinol. Metab. 75 (5): 1326–32. doi:10.1210/jc.75.5.1326. PMID 1430094.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gooren L (June 1989). "Improvement of spermatogenesis after treatment with the antiestrogen tamoxifen in a man with the incomplete androgen insensitivity syndrome". J. Clin. Endocrinol. Metab. 68 (6): 1207–10. doi:10.1210/jcem-68-6-1207. PMID 2566621.
External links
- Information
- Patient groups
- AIS Support Group AISSG (UK and International)
- AIS-DSD Support Group for Women & Families (US)
- AIS Support Group (Australasia)
- Intersex Support Forums (US and International)
This template is no longer used; please see Template:Endocrine pathology for a suitable replacement
