Pleistocene extinctions
It has been suggested that this article be merged into Pleistocene megafauna. (Discuss) Proposed since May 2007. |
The Pleistocene epoch saw the extinctions of numerous predominantly larger species, many of which occurred during the transition to the Holocene epoch in what is termed the Holocene extinction event. Among the main causes hypothesized by paleontologists are the spread of disease, natural climate change, and overkill by humans, which appeared during this epoch. A variant of this last possibility is the second-order predation hypothesis, which focuses more on the indirect damage caused by overcompetition with nonhuman predators.
Overkill Hypothesis

The Overkill hypothesis suggests that humans hunted megaherbivores to extinction. As a result, carnivores and scavengers that depended upon those animals became extinct from lack of prey.[1][2][3] The hypothesis was proposed 40 years ago by Paul S. Martin, now Professor of Geosciences Emeritus at the Desert Laboratory of the University of Arizona. It sparked debate which continues today. The most convincing evidence of his theory is that 80% of the North American large mammal species disappeared within 1000 years of the arrival of humans on the Western Hemisphere continents.

World wide extinctions seem to follow the migration of humans and to be most severe where humans arrived most recently and least severe where humans were originally – Africa (see figure at right). This suggests that in Africa, where humans evolved, prey animals and human hunting ability evolved together, so the animals evolved avoidance techniques. As humans migrated throughout the world and became more and more proficient at hunting, they encountered animals that had evolved without the presence of humans. Lacking the fear of humans that African animals had developed, animals outside of Africa were easy prey for human hunting techniques. It also suggests that this is independent of climate change (see figure at left).
Overkill has been supported by archaeological finds of mammoths with projectile points embedded in their skeletons, by observations of modern naïve animals allowing hunters to approach easily [4][5][6] and by computer models by Mosimann and Martin,[7]and Whittington and Dyke,[8] and most recently by Alroy.[9]
Shortcomings of the Overkill Hypothesis
The major objections to the theory are as follows:
- In predator-prey models it is unlikely that predators could over-hunt their prey since predators need their prey as food to sustain life and reproduce.[10]. This criticism has been rejected by many ecologists because humans have the widest dietary choice of any predator and are perfectly capable of switching to alternative prey or even plant foods when any prey species becomes rare. Humans have indisputably hunted numerous species to extinction, which renders any argument that human predators can never hunt prey to extinction immediately invalid.
- There is no evidence that megafauna other than mammoths, mastodons, and bison were hunted. (Meltzer) Overkill proponents, however, say this is due to chance and the low probability of animals with low populations to be fossilized. (Martin)
- A small number of animals that were hunted, such as a single species of bison, did not go extinct. However the surviving bison species in North America was a recent Eurasian acquisition that arrived in the Americas at approximately the same time as humans and was thus well-adapted to human hunting pressure. In contrast at least three endemic American bison species did become extinct. Bison also are r type herbivores and reproduce rapidly compared to animals that did go extinct such as proboscideans and horses.
- The dwarfing of animals is not explained by overkill. Numerous authors however have pointed out that dwarfing of animals is perfectly well explained by humans selectively harvesting the largest animals, and have provided proof that even within the 20th century numerous animal populations have reduced in average size due to human hunting.
- Eurasian Pleistocene megafauna went extinct in roughly same time period despite having much longer time to adapt to hunting pressure by humans.
- Hypothesis that Clovis culture were first humans to arrive in New World has been disputed recently. (See Models of migration to the New World)
Climate change hypotheses
At the end of the 19th and beginning of the 20th centuries, when scientists first realized that there had been glacial and interglacial ages, and that they were somehow associated with the prevalence or disappearance of certain animals, they surmised that the termination of the Pleistocene ice age might be an explanation for the extinctions.
Increased temperature
The most obvious change associated with the termination of an ice age is the increase in temperature. Between 15,000 BP and 10,000 BP, a 6°C increase in global mean annual temperatures occurred. This was generally thought to be the cause of the extinctions.
According to this hypothesis, a temperature increase sufficient to melt the Wisconsin ice sheet could have placed enough thermal stress on cold-adapted mammals to cause them to die. Their heavy fur, which helps conserve body heat in the glacial cold, might have prevented the dumping of excess heat, causing the mammals to die of heat exhaustion. Large mammals, with their reduced surface area-to-volume ratio, would have fared worse than small mammals.
Shortcomings of the Temperature Hypothesis
More recent research has demonstrated that the annual mean temperature of the current interglacial that we have seen for the last 10,000 years is no higher than that of previous interglacials, so the same large mammals survived similar temperature increases. Therefore warmer temperature alone is not a sufficient explanation.[11][12][13][14][15][16]
In addition, numerous species such as mammoths survived in human-free refugia such as Wrangel Island[17] despite changes in climate. This is precisely the opposite of what would be expected if climate change were responsible. Under normal ecological assumptions island populations should be more vulnerable to extinction due to climate change because of small populations and an inability to migrate to more favorable climes.
Increased continentality affects vegetation in time or space
Other scientists have proposed that increasingly extreme weather — hotter summers and colder winters — referred to as "continentality", or related changes in rainfall caused the extinctions. The various hypotheses are outlined below.
Vegetation changes: geographic
It has been shown that vegetation changed from mixed woodland-parkland to separate prairie and woodland.[13][14][16] This may have affected the kinds of food available. If so, herbivores might not have found the plants with which they had evolved and thus would have fallen prey to the anti-herbivory toxins in the plants that remained available. Shorter growing seasons may have caused the extinction of large herbivores and the dwarfing of many others. In this case, as observed, bison and other large ruminants would have fared better than horses, elephants and other monogastrics, because ruminants are able to extract more nutrition from limited quantities of high-fiber food and better able to deal with anti-herbivory toxins.[18][19][20] So, in general, when vegetation becomes more specialized, herbivores with less diet flexibility may be less able to find the mix of vegetation they need to sustain life and reproduce within a given area.
Rainfall changes: time
Increased continentality resulted in reduced and less predictable rainfall limiting the availability of plants necessary for energy and nutrition.[21][22][23] Axelrod[24] and Slaughter[25] have suggested that this change in rainfall restricted the amount of time favorable for reproduction. This could disproportionately harm large animals, since they have longer, more inflexible mating periods, and so may have produced young at unfavorable seasons (i.e., when sufficient food, water, or shelter was unavailable because of shifts in the growing season. In contrast, small mammals, with their shorter life cycles, shorter reproductive cycles, and shorter gestation periods, could have adjusted to the increased unpredictability of the climate, both as individuals and as species which allowed them to synchronize their reproductive efforts with conditions favorable for offspring survival. If so, smaller mammals would have lost fewer offspring and would have been better able to repeat the reproductive effort when circumstances once more favored offspring survival.[26]
Shortcomings of the continentality hypotheses
Critics have identified a number of problems with the continentality hypotheses.
- Megaherbivores have prospered at other times of continental climate. For example, megaherbivores thrived in Pleistocene Siberia, which had and has a more continental climate than Pleistocene or modern (post-Pleistocene, interglacial) North America.[27][28][29]
- The animals that went extinct actually should have prospered during the shift from mixed woodland-parkland to prairie, because their primary food source, grass, was increasing rather than decreasing.[30][31][29] Although the vegetation did become more spatially specialized, the amount of prairie and grass available increased, which would have been good for horses and for mammoths, and yet they went extinct.
- Although horses went extinct in the New World, they were successfully reintroduced by the Spanish in the 16th century – into a modern post-Pleistocene, interglacial climate. Today there are feral horses still living in those same environments. They find a sufficient mix of food to avoid toxins, they extract enough nutrition from forage to reproduce effectively and the timing of their gestation is not an issue. Similarly, mammoths survived the Pleistocene Holocene transition on isolated, uninhabited islands in the Mediterranean Sea[32] and on Wrangel Island in the Siberian Arctic [33] until 4,000 to 7,000 years ago.
- Large mammals should have been able to migrate, permanently or seasonally, if they found the temperature too extreme, the breeding season too short, or the rainfall too sparse or unpredictable.[34] Seasons vary geographically. By migrating away from the equator, herbivores could have found areas with growing seasons more favorable for finding food and breeding successfully. Modern-day African elephants migrate during periods of drought to places where there is apt to be water.[35]
- Large animals store more fat in their bodies than do medium-sized animals[36] and this should have allowed them to compensate for extreme seasonal fluctuations in food availability.
The extinction of the megafauna could have caused the extinction of the mammoth steppe. Alaska now has low nutrient soil unable to support bison, mammoths, and horses. R. Dale Guthrie has claimed this as a cause of the extinction of the megafauna there, hoever he may be interpeting it backwards. Chapin (Chapin 1980) showed that simply adding fertilizer to the soil in Alaska could make grasses grow again like they did in the era of the mammoth steppe. Possibly, the extinction of the megafauna and the correspoding loss of dung is what led to low nutrient levels in modern day soil and therefore is why the landscape can no longer support a megafauna.
Shortcomings of both Climate Change and Overkill
Neither the Overkill sensu stricto nor Climate Change hypotheses explain several observations.
- Browsers, mixed feeders and non-ruminant grazer species suffered most, while ruminant grazers generally survived. However a broader scope of overkill predicts this perfectly because changes in vegetation wrought by anthropogenic fire preferentially selects against browse species.
- Many surviving mammal species were sharply diminished in size, a fact which many authors have pointed out perfectly fits the Overkill Hypothesis and is reflected in the dwarfing of many hunted species even within the 20th century.
Because of the unsatisfactory nature of the Overkill or Climate Change hypotheses alone many scientists support some combination of Climate Change and Overkill.
Hyperdisease Hypothesis
Theory
The Hyperdisease Hypothesis attributes the extinction of large mammals during the late Pleistocene to indirect effects of the newly arrived aboriginal humans.[37][38] The Hyperdisease Hypothesis proposes that humans or animals traveling with them (e.g., domestic dogs) introduced one or more highly virulent diseases into vulnerable populations of native mammals, eventually causing extinctions. The extinction was biased toward larger-sized species because smaller species have greater resilience because of their life history traits (e.g., shorter gestation time, greater population sizes, etc). Humans are thought to be the cause because other earlier immigrations of mammals into North America from Eurasia did not cause extinctions.[37]
If a disease was indeed responsible for the end-Pleistocene extinctions, then there are several criteria it must satisfy (see Table 7.3 in MacPhee & Marx 1997). First, the pathogen must have a stable carrier state in a reservoir species. That is, it must be able to sustain itself in the environment when there are no susceptible hosts available to infect. Second, the pathogen must have a high infection rate, such that it is able to infect virtually all individuals of all ages and sexes encountered. Third, it must be extremely lethal, with a mortality rate of c. 50–75%. Finally, it must have the ability to infect multiple host species without posing a serious threat to humans. Humans may be infected, but the disease must not be highly lethal or able to cause an epidemic.
Shortcomings of the Hyperdisease Hypothesis
- No evidence of disease has been found.
- Generally speaking, disease has to be very virulent to kill off all the individuals in a genus or species. Even such a virulent disease as West Nile Virus is unlikely to have caused extinction.[39]
- The disease would need to be implausibly selective while being simultaneously implausibly broad. Such a disease needs to be capable of killing of three species of bison while leaving a third very closely related species unaffected. It would need to be capable of killing off flightless birds while leaving closely related flighted species unaffected. Yet while remaining sufficiently selective to afflict only individual species within genera it must be capable of fatally infecting across such clades as birds, marsupials, placentals, testudines, and crocodilians. No disease with such a broad scope of fatal infectivity is known, much less one that remains simultaneously incapable of infecting numerous closely related species within those disparate clades.
Second-Order Predation

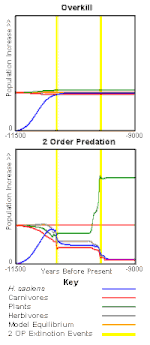
Scenario
The Second-Order Predation Hypothesis says that as humans entered the New World they continued their policy of killing predators, which upset the ecological balance of the continent causing overpopulation, environmental exhaustion, and environmental collapse. The hypothesis accounts for changes in animal, plant, and, human populations.
The scenario is as follows:
- After the arrival of H. sapiens in the New World, existing predators must share the prey populations with this new predator. Because of this competition, populations of original, or first-order, predators cannot find enough food they are in direct competition with humans.
- Second-order predation begins as humans begin to kill predators.
- Prey populations are no longer well controlled by predation. Killing of nonhuman predators by H. sapiens reduces their numbers to a point where these predators no longer regulate the size of the prey populations.
- Lack of regulation by first-order predators triggers boom-and-bust cycles in prey populations. Prey populations expand and consequently overgraze and over-browse the land. Soon the environment is no longer able to support them. As a result, many herbivores starve. Species that rely on the slowest recruiting food become extinct, followed by species that cannot extract the maximum benefit from every bit of their food.
- Boom-bust cycles in herbivore populations change the nature of the vegetative environment, with consequent climatic impacts on relative humidity and continentality. Through overgrazing and overbrowsing, mixed parkland becomes grassland, and climatic continentality increases.
Support
This has been supported by a computer model, the Pleistocene Extinction Model (PEM), which, using the same assumptions and values, compares hypotheses with Second-Order Predation. The findings are that Second Order-Predation is more consistent with extinction than is Overkill[40] (results graph at left). The PEM was run to test combination hypotheses by artificially introducing sufficient climate change to cause extinction. When Overkill and Climate Change are combined they balance each other out. Climate Change reduces the number of plants, Overkill removes animals, therefore fewer plants are eaten. Second-Order Predation combined with Climate Change exacerbates the extinction[41] (results graph at right).
Second-Order Predation and other theories
- Climate Change: Second-Order Predation accounts for the changes in vegetation, which in turn may account for the increase in continentality. Since the extinction is due to destruction of habitat it accounts for the loss of animals not hunted by humans. Second-Order Predation accounts for the dwarfing of animals as well as extinctions since animals that could survive and reproduce on less food would be selectively favored.
- Hyperdisease: The reduction of carnivores could have been from distemper or other carnivore disease carried by domestic dogs.
- Overkill: The observation that extinctions follow the introduction of humans is supported by the Second-Order Predation hypothesis.
Shortcomings of the Second-Order Predation Hypothesis
- No evidence of humans hunting predators has been found in the New World though it has been found in Siberia.[42]
- Like all climate-based theories, the model predicts large extinctions in response to climate change and without human hunting, so it is unable to explain why these extinctions did not occur during numerous deglaciations of equal intensity, or why they did not occur at high latitudes in Eurasia.
- It assumes decreases in vegetation due to climate change, but deglaciation doubled the habitable area of North America.
- Climate change had little effect on vegetation or the distribution of small mammals, reptiles, and amphibians throughout the southern half of the United States, not to mention tropical regions throughout the Americas that also suffered catastrophic extinctions.
- Any vegetational changes that did occur failed to cause almost any extinctions of small vertebrates, and they are more narrowly distributed on average.
- The model specifically assumes high extinction rates in grasslands, but most extinct species ranged across numerous vegetation zones, historical population densities of ungulates were very high in the Great Plains, these environments support high ungulate diversity throughout Africa, and extinction intensity was equally severe in forested environments.
- It is unable to explain why large herbivore populations were not regulated by surviving carnivores such as grizzly bears, wolves, pumas, and jaguars whose populations would have increased rapidly in response to the loss of competitors.
- It does not explain why almost all extinct carnivores were large herbivore specialists such as sabre toothed cats and short faced bears, but most hypocarnivores and generalized carnivores survived.
- There is no historical evidence of boom and bust cycles causing even local extinctions in regions where large mammal predators have been driven extinct by hunting. The recent hunting out of remaining predators throughout most of the United States has not caused massive vegetational change or dramatic boom and bust cycles in ungulates.
- It is not spatially explicit and does not track predator and prey species separately, whereas the multispecies overkill model does both.
- The multispecies model produces a mass extinction through indirect competition between herbivore species: small species with high reproductive rates subsidize predation on large species with low reproductive rates.[9] The fact that all prey species are lumped in the Pleistocene Extinction Model explains why it performs poorly without adding extra assumptions about climate change and cascade effects.
- Everything explained by this model also is explained by the multispecies model, but with less assumptions, so this one is not parsimonious.
References
- ^ Martin P. S. (1963). The last 10,000 years: A fossil pollen record of the American Southwest. Tucson, AZ: Univ. Ariz. Press. ISBN 0-8165-1759-2.
- ^ Martin P. S. (1967). Prehistoric overkill. In Pleistocene extinctions: The search for a cause (ed. P.S. Martin and H.E. Wright). New Haven: Yale Univ. Press. ISBN 0-300-00755-8.
- ^ Martin P. S. (1989). Prehistoric overkill: A global model. In Quaternary extinctions: A prehistoric revolution (ed. P.S. Martin and R.G. Klein). Tucson, AZ: Univ. Arizona Press. pp. 354–404. ISBN 0-8165-1100-4.
- ^ Flannery, T (1995). The future eaters: an ecological history of the Australasian lands and people. NY: George Braziller. ISBN 0-8021-3943-4.
- ^ Diamond, J. (1984). Historic extinctions: a Rosetta stone for understanding prehistoric extinctions. In Quaternary extinctions: A prehistoric revolution (ed. P.S. Martin and R.G. Klein). Tucson, AZ: Univ. Arizona Press. pp. 824–62. ISBN 0-8165-1100-4.
- ^ Diamond, J. (1997). Guns, germs, and steel; the fates of human societies. New York: Norton. ISBN 0-393-31755-2.
- ^ Mossiman, J. E., and Martin, P. S. (1975). "Simulating Overkill by Paleoindians". American Scientist. 63: 304–13.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Whittington, S. L. & Dyke, B. (1984). Simulating overkill: experiment with the Mossiman and Martin model. In Quaternary extinctions: A prehistoric revolution (ed. P.S. Martin and R.G. Klein). Tucson, AZ: Univ. Arizona Press. pp. 451–66. ISBN 0-8165-1100-4.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b Alroy, J. (2001). "A multispecies overkill simulation of the end-Pleistocene megafaunal mass extinction" (PDF). Science. 292: 1893. doi:10.1126/science.1059342.
- ^ May, R. M. (2001). Stability and complexity in model ecosystems. Princeton: Princeton Univ. Press. ISBN 0-691-08861-6.
- ^ Andersen, S. T (1973). The differential pollen productivity of trees and its significance for the interpretation of a pollen diagram from a forested region. In Quaternary plant ecology: the 14thsymposium of the British Ecological society, University of Cambridge, 28–30 March 1972 (ed. Birks, H. J. B. and West, R. G). Oxford: Blackwell Scientific Pubs. ISBN 0-632-09120-7.
- ^ Ashworth, C.A. (1980). "Environmental implications of a beetle assemblage from the Gervais formation (Early Wisconsinian?), Minnesota". Quat. Res. 13: 200–12. doi:10.1016/0033-5894(80)90029-0.
- ^ a b Birks, H. H. (1973). Modern macrofossil assemblages in lake sediments in Minnesota. In Quaternary plant ecology: the 14thsymposium of the British Ecological Society, University of Cambridge, 28–30 March 1972 (ed. H. J. B. Birks, and R. G. West). Oxford: Blackwell Scientific Pubs. ISBN 0-632-09120-7.
- ^ a b Birks, H. J. B. & Birks, H. H. (1980). Quaternary paleoecology. Baltimore: Univ. Park Press. ISBN 1-930665-56-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Bradley, R. S. (1985). Quaternary Paleoclimatology: Methods of Paleoclimatic Reconstruction. Winchester, MA: Allen & Unwin. ISBN 0-04-551068-7.
- ^ a b Davis, M. B. (1976). Pleistocene biogeography of temperate deciduous forests. In Geoscience and man: ecology of the Pleistocene, vol.13. Baton Rouge: School of Geoscience, Louisiana State Univ.
- ^ Vartanyan, S.L., Arslanov, K.A., Tertychnaya, T.V. & Chernov, S.B. (1995). "Radiocarbon dating evidence for mammoths on Wrangel Island, Arctic Ocean, until 2000 BC". Radiocarbon. 37: 1–6.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Guthrie, R. D. (1988). Frozen Fauna of the Mammoth Steppe: The Story of Blue Babe. University Of Chicago Press. ISBN 0-226-31122-8.
- ^ Guthrie, R. D. (1989). Mosaics, allochemics, and nutrients: an ecological theory of Late Pleistocene megafaunal extinctions. In Quaternary extinctions: A prehistoric revolution (ed. P.S. Martin and R.G. Klein). Tucson, AZ: Univ. Arizona Press. pp. 259–99. ISBN 0-8165-1100-4.
- ^ Hoppe, P. P. (1978), Rumen fermentation in African ruminants. Proceedings of the 13th Annual Congress of Game Biologists, Atlanta
{{citation}}: CS1 maint: location missing publisher (link) - ^ Bryson, R. A., Baerreis, D. A. & Wendland, W. M. (1970). The character of late-glacial and post-glacial climatic changes. Pleistocene and recent environments of the central Great Plains (ed. W. Dort, Jr. and J. K. Jones, Jr., Dept. Geol., Univ. Kan. Spec. Pub. 3). Lawrence: Univ. Press Kan. ISBN 0-7006-0063-9.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Graham, R.W. & Lundelius, E.L. (1989). Coevolutionary disequilibrium and Pleistocene extinctions. In Quaternary extinctions: A prehistoric revolution (ed. P.S. Martin and R.G. Klein). Tucson, AZ: Univ. Arizona Press. pp. 354–404. ISBN 0-8165-1100-4.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ King, J. E. and Saunders, J. J. (1989). Environmental insularity and the extinction of the American mastodont. In Quaternary extinctions: A prehistoric revolution (ed. P.S. Martin and R.G. Klein). Tucson, AZ: Univ. Arizona Press. pp. 354–404. ISBN 0-8165-1100-4.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Axelrod, D. I. (1967). "Quaternary extinctions of large mammals". University of California Publications in Geological Sciences. 74: 1–42. ASIN B0006BX8LG.
- ^ Slaughter, B. H. (1967). Animal ranges as a clue to late-Pleistocene extinction. In Pleistocene extinctions: The search for a cause (ed. P.S. Martin and H.E. Wright). New Haven: Yale Univ. Press. ISBN 0-300-00755-8.
- ^ Kilti, R. A. (1988). Seasonality, gestation time, and large mammal extinctions. In Quaternary extinctions: A prehistoric revolution (ed. P.S. Martin and R.G. Klein). Tucson, AZ: Univ. Arizona Press. pp. 354–404. ISBN 0-8165-1100-4.
- ^ Flereov, C. C. (1967). On the origin of the mammalian fauna of Canada. In The Bering Land Bridge (ed. D.M. Hopkins). Palo Alto: Stanford Univ. Press. pp. 271–80. ISBN 0-8047-0272-1.
- ^ Frenzel, B. (1968). "The Pleistocene vegetation of northern Eurasia". Science. 161: 637–49. doi:10.1126/science.161.3842.637.
- ^ a b McDonald, J. (1989). The reordered North American selection regime and late Quaternary megafaunal extinctions. In Quaternary extinctions: A prehistoric revolution (ed. P.S. Martin and R.G. Klein). Tucson, AZ: Univ. Arizona Press. pp. 354–404. ISBN 0-8165-1100-4.
- ^ Birks, H. J. B. and West, R. G. (1973). Quaternary plant ecology: the 14th symposium of the British Ecological society, University of Cambridge, 28–30 March 1972. Oxford: Blackwell Scientific Pubs. ISBN 0-632-09120-7.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ McDonald, J. (1981). North American Bison: Their classification and evolution. Berkeley: Univ. Calif. Press. ISBN 0-520-04002-3.
- ^ Burney, D. A. (1993). "Recent animal extinctions: recipes for disaster". American Scientist. 81 (6): 530–41.
- ^ Vartanyan, S.L., Garutt, V. E. and Sher, A.V. (1993). "Holocene dwarf mammoths from Wangel Island in the Siberian Arctic". Nature. 362: 337–40. doi:10.1038/362337a0.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pennycuick, C.J. (1979). Energy costs of locomotion and the concept of "Foraging radius." In Serengetti: Dynamics of an Ecosystem (ed. A.R.E. Sinclair and M. Norton-Griffiths). Chicago: Univ. Chicago Press. pp. 164–85. ISBN 0-226-76029-4.
- ^ Wing, L.D. & Buss, I.O. (1970). "Elephants and Forests". Wildl. Mong. (19).
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Owen-Smith, R.N. (1992). Megaherbivores: The influence of very large body size on ecology. Cambridge studies in ecology. Cambridge: Cambridge Univ. Press. ISBN 0-521-42637-5.
- ^ a b MacFee, R.D.E. & Marx, P.A. (1997). Humans, hyperdisease and first-contact extinctions. In Natural Change and Human Impact in Madagascar (eds S. Goodman & B.D. Patterson). Washington D.C.: Smithsonian Press. pp. 169–217. ISBN 1-56098-683-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ MacFee, R.D.E. & Marx, P.A. (1998). "Lightning Strikes Twice: Blitzkrieg, Hyperdisease, and Global Explanations of the Late Quaternary Catastrophic Extinctions". American Museum of Natural History.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Lyons, K, Smith, F.A., Wagner, P.J., White, E.P., and Brown, J.,H. (2004). "Was a 'hyperdisease' responsible for the late Pleistocene megafaunal extinction?" ([dead link] – Scholar search). Ecology. 7: 859–68.
{{cite journal}}: External link in|format= - ^ Whitney-Smith, E. (2004). Late Pleistocene extinctions through second-order predation. In Settlement of the American Continents: A Multidisciplinary Approach to Human Biogeography (eds C. M. Barton, G. A. Clark, D. R. Yesner). Tucson, AZ: University of Arizona Press. ISBN 0-8165-2323-1.
- ^ Whitney-Smith, E. (2006). Clovis and Extinctions – Overkill, Second Order Predation, Environmental Degradation in a Non-equilibrium Ecosystem "Clovis Age Continent". University of New Mexico Press.
- ^ Soffer, O. (1985). The Upper Paleolithic of the Central Russian Plain. Orlando, Florida: Academic Press. ISBN 0-12-654270-8.
External links
Hyperdisease Hypothesis
- American Museum of Natural History
- J.H. Brown (University of New Mexico). Was a hyperdisease responsible?
