Podocyte
| Podocyte | |||
|---|---|---|---|
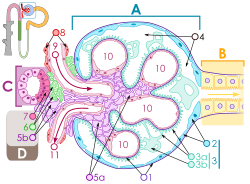 Renal corpuscle structure
Blood flows in the afferent arteriole (9) at the top, and out the efferent arteriole (11) at the bottom. Blood flows through the capillaries of the glomerulus (10), where it is filtered by pressure. The podocytes (3a and 3b, green) are wrapped around the capillaries. Blood is filtered through the slit diaphragm (or filtration slit), between the feet or processes of the podocytes. The filtered blood passes out the proximal tubule (B, yellow) on the right.
| |||
| Details | |||
| Precursor | Intermediate mesoderm | ||
| Identifiers | |||
| Latin | podocytus | ||
| MeSH | D050199 | ||
| FMA | 70967 | ||
| Anatomical terminology | |||
Podocytes are cells in the Bowman's capsule in the kidneys that wrap around capillaries of the glomerulus.[1] The Bowman's capsule filters the blood, retaining large molecules such as proteins while smaller molecules such as water, salts, and sugars are filtered as the first step in the formation of urine. Although various viscera have epithelial layers, the name visceral epithelial cells usually refers specifically to podocytes, which are specialized epithelial cells that reside in the visceral layer of the capsule.
The podocytes have long processes, called foot processes, foot projections, or pedicels, for which the cells are named (podo- + -cyte). The foot projections wrap around the capillaries and leave slits between them. Blood is filtered through these slits, each known as a filtration slit or slit diaphragm. Several proteins are required for the foot projections to wrap around the capillaries and function. When infants are born with certain defects in these proteins, such as nephrin and CD2AP, their kidneys cannot function. People have variations in these proteins, and some variations may predispose them to kidney failure later in life. Nephrin is a zipper-like protein that forms the slit diaphragm, with spaces between the teeth of the zipper, big enough to allow sugar and water through, but too small to allow proteins through. Nephrin defects are responsible for congenital kidney failure. CD2AP regulates the podocyte cytoskeleton and stabilizes the slit diaphragm.[2][3]
Structure

Podocytes are found lining the Bowman's capsules in the nephrons of the kidney. The foot processes known as pedicels that extend from the podocytes wrap themselves around the capillaries of the glomerulus to form the filtration slits. The pedicels increase the surface area of the cells enabling efficient ultrafiltration.[4]
There are numerous coated vesicles and coated pits along the basolateral domain of the podocytes which indicate a high rate of vesicular traffic.
Podocytes possess a well-developed endoplasmic reticulum and a large Golgi apparatus, indicative of a high capacity for protein synthesis and post-translational modifications.
There is also growing evidence of a large number of multivesicular bodies and other lysosomal components seen in these cells, indicating a high endocytic activity.
Function

A. The endothelial cells of the glomerulus; 1. pore (fenestra).
B. Glomerular basement membrane: 1. lamina rara interna 2. lamina densa 3. lamina rara externa
C. Podocytes: 1. enzymatic and structural protein 2. filtration slit 3. diaphragma
Adjacent podocytes interdigitate to cover the basal lamina which is intimately associated with the glomerular capillaries. The pedicels of the podocytes interdigitate and leave gaps or thin filtration slits between them. The slits are covered by slit diaphragms which are composed of a number of cell-surface proteins including nephrin, podocalyxin, and P-cadherin, which restrict the passage of large macromolecules such as serum albumin and gamma globulin and ensure that they remain in the bloodstream.[5] Proteins that are required for the correct function of the slit diaphragm include nephrin,[6] NEPH1, NEPH2,[7] podocin, and CD2AP.[8]
Small molecules such as water, glucose, and ionic salts are able to pass through the filtration slits and form an ultrafiltrate in the tubular fluid, which is further processed by the nephron to produce urine.
Podocytes are also involved in regulation of glomerular filtration rate (GFR). When podocytes contract, they cause closure of filtration slits. This decreases the GFR by reducing the surface area available for filtration.
Clinical significance
A Loss of the foot processes of the podocytes (i.e., podocyte effacement) is a hallmark of minimal change disease, which has therefore sometimes been called foot process disease.
Disruption of the filtration slits or destruction of the podocytes can lead to massive proteinuria, where large amounts of protein are lost from the blood.
An example of this occurs in the congenital disorder Finnish-type nephrosis, which is characterised by neonatal proteinuria leading to end-stage renal failure. This disease has been found to be caused by a mutation in the nephrin gene.
References
- ^ "Podocyte" at Dorland's Medical Dictionary
- ^ Wickelgren, I. (1999). "CELL BIOLOGY: First Components Found for Key Kidney Filter". Science. 286 (5438): 225–6. doi:10.1126/science.286.5438.225. PMID 10577188.
- ^ Löwik MM, Groenen PJ, Levtchenko EN, Monnens LA, van den Heuvel LP (November 2009). "Molecular genetic analysis of podocyte genes in focal segmental glomerulosclerosis—a review". Eur. J. Pediatr. 168: 1291–304. doi:10.1007/s00431-009-1017-x. PMC 2745545. PMID 19562370.
- ^ Template:GeorgiaPhysiology
- ^ Jarad, G.; Miner, J. H. (2009). "Update on the glomerular filtration barrier". Current opinion in nephrology and hypertension. 18 (3): 226–232. doi:10.1097/mnh.0b013e3283296044. PMC 2895306. PMID 19374010.
- ^ Wartiovaara, J.; Ofverstedt, L. G. R.; Khoshnoodi, J.; Zhang, J.; Mäkelä, E.; Sandin, S.; Ruotsalainen, V.; Cheng, R. H.; Jalanko, H.; Skoglund, U.; Tryggvason, K. (2004). "Nephrin strands contribute to a porous slit diaphragm scaffold as revealed by electron tomography". Journal of Clinical Investigation. 114 (10): 1475–1483. doi:10.1172/JCI22562. PMC 525744. PMID 15545998.
- ^ Neumann-Haefelin, E.; Kramer-Zucker, A.; Slanchev, K.; Hartleben, B.; Noutsou, F.; Martin, K.; Wanner, N.; Ritter, A.; Gödel, M.; Pagel, P.; Fu, X.; Müller, A.; Baumeister, R.; Walz, G.; Huber, T. B. (2010). "A model organism approach: Defining the role of Neph proteins as regulators of neuron and kidney morphogenesis". Human Molecular Genetics. 19 (12): 2347–2359. doi:10.1093/hmg/ddq108. PMID 20233749.
- ^ Fukasawa, H.; Bornheimer, S.; Kudlicka, K.; Farquhar, M. G. (2009). "Slit Diaphragms Contain Tight Junction Proteins". Journal of the American Society of Nephrology. 20 (7): 1491–1503. doi:10.1681/ASN.2008101117. PMC 2709684. PMID 19478094.
External links
- Anatomy photo: Urinary/mammal/vasc1/vasc1 - Comparative Organology at University of California, Davis - "Mammal, renal vasculature (EM, High)
- Histology image: 22401loa – Histology Learning System at Boston University - ". Ultrastructure of the Cell: podocytes and glomerular capillaries"
- UIUC Histology Subject 1400
- podocyte.ca at Samuel Lunenfeld Research Institute
- Template:GeorgiaPhysiology
- Histology image: 22402loa – Histology Learning System at Boston University
- Histology image: 22403loa – Histology Learning System at Boston University
