Podophyllotoxin
 | |
| Clinical data | |
|---|---|
| Other names | (5R,5aR,8aR,9R)-9-hydroxy-5-(3,4,5-trimethoxyphenyl)-5,8,8a,9-tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-6(5aH)-one |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a684055 |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 1.0 to 4.5 hours. |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.502 |
| Chemical and physical data | |
| Formula | C22H22O8 |
| Molar mass | 414.405 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Podophyllotoxin (abbreviated as PPT), otherwise known as podofilox, is a non-alkaloid toxin lignan extracted from the roots and rhizomes of Podophyllum species.[1] Under the trade names Condylox, a gel, and Wartec, a solution or cream, it is used on the skin as a topical treatment of external genital warts, caused by some types of the human papillomavirus (HPV), and other warts. PPT and its derivatives display a wide selection in medical applications such as purgative, vesicant, antirheumatic, antiviral, and antitumor agents. These derivatives include etoposide, teniposide, and etopophos. Their anticancer activity has been heavily under study and used in various chemotherapies, including lung cancer, lymphomas, and genital tumors.
Natural abundance
It is present at concentrations of 0.3 to 1.0% by mass in the rhizome of American Mayapple (Podophyllum peltatum).[2][3] Another common source of podophyllotoxin is the rhizomes of Podophyllum hexandrum Royle (Berberidaceae).
It is biosynthesized from two molecules of coniferyl alcohol by phenolic oxidative coupling and a series of oxidations, reductions and methylations.[2]
Structural characteristic
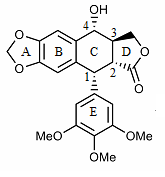
The structure of podophyllotoxin was first elucidated in the 1930s.[4] Podophyllotoxin bears a four consecutive chiral centers, labelled C-1 through C-4. The molecule also contains four almost planar fused rings. Four ends of podophyllotoxin have oxygen atoms at the functional groups dioxoles, methoxys, lactone, and secondary alcohol.[5]
Derivatives of podophyllotoxin are synthesized as properties of the rings and carbon 1 through 4 are diversified. For example, ring A is not essential to antimitotic activity. Aromatization of ring C leads to loss of activity, possibly from ring E no longer being placed on the axial position. In addition, the stereochemistry at C-2 and C-3 configures a trans-lactone, which has more activity than the cis counterpart. Chirality at C-1 is also important as it implies an axial position for ring E.[5]
Biosynthesis
Although the biosynthetic route of podophyllotoxin has yet to be completely elucidated, several studies have suggested a common pathway starting from coniferyl alcohol being converted to (+)-pinoresinol in the presence of a one-electron oxidant [6] through dimerization of stereospecific radical intermediate. Pinoresinol is subsequently reduced in the presence of co-factor NADPH to first lariciresinol, and ultimately secoisolariciresinol. Lactonization on secoisolariciresinol gives rise to matairesinol. Secoisolariciresinol is assumed to be converted to yatein through appropriate quinomethane intermediates,[6] leading to podophyllotoxin.
Proposed biosynthetic pathway leading to podophyllotoxin.
Side effects
Application can be immediately followed by burning or itching. Small sores, itching and peeling skin can also follow.[7]
Usages and applications
Podophyllotoxin displays a range of activities such as cathartic, purgative, antiviral, vesicant, and antihelminthic. Additionally, the lignan and its derivatives are exciting leads for anti-tumor agent. For instance, podophyllotoxin is the pharmacological precursor for the important anticancer drug etoposide.[6][8]
Mechanism of action
Etoposide, a semisynthetic derivative of podophyllotoxin, induces DNA breakage through its inhibition of topoisomerase II. The drug is most active in the late S and early G2 phases of the cell cycle. Teniposide is an analog with very similar pharmacologic characteristics. [9]
Podophyllotoxin derivatives display binding activity to the enzyme topoisomerase II during the late S and early G2 stage. For instance, etoposide binds and stabilizes the temporary break caused by the enzyme, disrupts the reparation of the break through which the double-stranded DNA passes, and consequently stops DNA unwinding and replication.[2] Mutants resistant to either podophyllotoxin, or to its topoisomerase II inhibitory derivatives such as etoposide (VP-16), have been described in Chinese hamster cells.[10][11] The mutually exclusive cross-resistance patterns of these mutants provide a highly specific mean to distinguish the two kinds of podophyllotoxin derivatives.[11][12] Mutant Chinese hamster cells resistant to podophyllotoxin are affected in a protein P1 that was later identified as the mammalian HSP60 or chaperonin protein.[13][14]
References
- ^ Xu, H; Lv, M; Tian,X (2009). "A review on hemisynthesis, biosynthesis, biological activities, mode of action, and structure-activity relationship of podophyllotoxins: 2003-2007". Current Medicinal Chemistry. 16 (3): 327–349. doi:10.2174/092986709787002682. PMID 19149581.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Canel, C; Moraes, RM; Dayan, FE; Ferreira, D (2000). "Molecules of Interest: Podophyllotoxin". Phytochemistry. 54 (2): 115–120.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ J. L. Hartwell, A. W. Schrecker (1951). "Components of Podophyllin. V. The Constitution of Podophyllotoxin". Journal of the American Chemical Society. 73 (6): 2909–2916. doi:10.1021/ja01150a143.
- ^ Borsche, W.; Niemann J. (1932). "Über Podophyllin". Justus Liebigs Ann. Chem. 494: 126–142. doi:10.1002/jlac.19324940113.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b You, Y (2005). "Podophyllotoxin derivatives: current synthetic approaches for new anticancer agents". Current Pharmaceutical Design. 11 (13): 1695–1717. doi:10.2174/1381612053764724. PMID 15892669.
- ^ a b c Gordaliza M, García PA, del Corral JM, Castro MA, Gómez-Zurita MA (2004). "Podophyllotoxin: distribution, sources, applications and new cytotoxic derivatives". Toxicon. 44 (4): 441–59. doi:10.1016/j.toxicon.2004.05.008. PMID 15302526.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "PRODUCT INFORMATION WARTEC® SOLUTION" (PDF). GlaxoSmithKline Australia Pty Ltd. Retrieved 6 January 2013.
- ^ Damayanthi Y, Lown JW (June 1998). "Podophyllotoxins: current status and recent developments". Curr. Med. Chem. 5 (3): 205–52. PMID 9562603.
- ^ Anthony J. Trevor, Bertram G. Katzung, Marieke Kruidering-Hall, Susan B. Masters. Chapter 54: Cancer Chemotherapy. Katzung & Trevor's Pharmacology: Examination & Board Review, 10th edition.
- ^ Gupta, R.S., Ho, T.K.W., Moffat, M.R.K. and Gupta, R. (1982). Podophyllotoxin-resistant mutants of Chinese hamster ovary cells. Alteration in a microtubule-associated protein. J. Biol. Chem. 257: 1071-1078. [Abstract]
- ^ a b Gupta, R.S. (1983). Genetic, biochemical and cross-resistance studies with mutants of Chinese hamster ovary cells resistant to the anticancer drugs VM-26 and VP16-213. Cancer Res. 43: 1568-1574. [Abstract]
- ^ Gupta, R.S. (1983). Podophyllotoxin resistant mutants of Chinese hamster ovary cells: Cross resistance studies with various microtubule inhibitors and podophyllotoxin analogs. Cancer Res. 43: 505-512. [Abstract]
- ^ Picketts, D.J., Mayanil, C.S.K. and Gupta, R.S. (1989). Molecular cloning of a Chinese hamster mitochondrial protein related to the "chaperonin" family of bacterial and plant proteins. J. Biol. Chem. 264: 12001-12008. [Abstract]
- ^ Jindal, S., Dudani, A.K., Singh, B., Harley, C.B. and Gupta, R.S (1989). Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65 kDa mycobacterial antigen. Mol. Cell Biol. 9: 2279-2283. [Abstract]