Titanium: Difference between revisions
L Kensington (talk | contribs) m Reverted addition of dubious unsourced content (HG) |
Tick avenger (talk | contribs) mNo edit summary |
||
| Line 2: | Line 2: | ||
{{pp-move-indef}} |
{{pp-move-indef}} |
||
{{Infobox titanium}} |
{{Infobox titanium}} |
||
'''Titanium''' ({{IPAc-en|icon|t|aɪ|ˈ|t|eɪ|n|i|əm}} {{respell|tye|TAY|nee-əm}}) is a [[chemical element]] with the symbol '''Ti''' and [[atomic number]] 22. Sometimes called the "space age metal",<ref>{{cite book | title = Chemistry: Principles and Reactions | author1 = William L. Masterton | author2 = Cecile N. Hurley | edition = 6th | publisher = Cengage Learning | year = 2008 | isbn = 0495126713 | page = 18 | url = http://books.google.com/?id=teubNK-b2bsC&pg=PT44&lpg=PT44&dq=titanium+%22space-age+metal%22&q=titanium%20%22space-age%20metal%22 }}</ref> it has a low density and is a strong, lustrous, [[corrosion]]-resistant (including [[sea water]], [[aqua regia]] and [[chlorine]]) [[transition metal]] with a silver color. |
'''Titanium''' ({{IPAc-en|icon|t|aɪ|ˈ|t|eɪ|n|i|əm}} {{respell|tye|TAY|nee-əm}}) is a [[chemical element]] with the symbol '''Ti''' and [[atomic number]] 22. Sometimes called the "space age metal",<ref>{{cite book | title = Chemistry: Principles and Reactions | author1 = William L. Masterton | author2 = Cecile N. Hurley | edition = 6th | publisher = Cengage Learning | year = 2008 | isbn = 0495126713 | page = 18 | url = http://books.google.com/?id=teubNK-b2bsC&pg=PT44&lpg=PT44&dq=titanium+%22space-age+metal%22&q=titanium%20%22space-age%20metal%22 }}</ref> it has a low density and is a strong, lustrous, [[corrosion]]-resistant (including [[sea water]], [[aqua regia]] and [[chlorine]]) [[transition metal]] with a silver color and is closely related to marijuana. |
||
Titanium was discovered in England by [[William Gregor]] in 1791 and named by [[Martin Heinrich Klaproth]] for the [[Titan (mythology)|Titans]] of [[Greek mythology]]. |
Titanium was discovered in England by [[William Gregor]] in 1791 and named by [[Martin Heinrich Klaproth]] for the [[Titan (mythology)|Titans]] of [[Greek mythology]]. |
||
Revision as of 13:32, 19 September 2010
 | |||||||||||||||||||||||||||||||||||||||||
| Titanium | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | |||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery grey-white metallic | ||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Ti) | |||||||||||||||||||||||||||||||||||||||||
| Titanium in the periodic table | |||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 22 | ||||||||||||||||||||||||||||||||||||||||
| Group | group 4 | ||||||||||||||||||||||||||||||||||||||||
| Period | period 4 | ||||||||||||||||||||||||||||||||||||||||
| Block | d-block | ||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d2 4s2 | ||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 10, 2 | ||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||
| Melting point | 1941 K (1668 °C, 3034 °F) | ||||||||||||||||||||||||||||||||||||||||
| Boiling point | 3560 K (3287 °C, 5949 °F) | ||||||||||||||||||||||||||||||||||||||||
| Density (at 20° C) | 4.502 g/cm3 [4] | ||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 4.11 g/cm3 | ||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 14.15 kJ/mol | ||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 425 kJ/mol | ||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 25.060 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −2, −1, 0,[5] +1, +2, +3, +4[6] (an amphoteric oxide) | ||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.54 | ||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 147 pm | ||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 160±8 pm | ||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal close-packed (hcp) (hP2) | ||||||||||||||||||||||||||||||||||||||||
| Lattice constants | a = 295.05 pm c = 468.33 pm (at 20 °C)[4] | ||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 9.68×10−6/K (at 20 °C)[a] | ||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 21.9 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 420 nΩ⋅m (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | ||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | +153.0×10−6 cm3/mol (293 K)[7] | ||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 116 GPa | ||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 44 GPa | ||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 110 GPa | ||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 5090 m/s (at r.t.) | ||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.32 | ||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 6.0 | ||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 830–3420 MPa | ||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 716–2770 MPa | ||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-32-6 | ||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||
| Discovery | William Gregor (1791) | ||||||||||||||||||||||||||||||||||||||||
| First isolation | Jöns Jakob Berzelius (1825) | ||||||||||||||||||||||||||||||||||||||||
| Named by | Martin Heinrich Klaproth (1795) | ||||||||||||||||||||||||||||||||||||||||
| Isotopes of titanium | |||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||
Titanium (/[invalid input: 'icon']taɪˈteɪniəm/ tye-TAY-nee-əm) is a chemical element with the symbol Ti and atomic number 22. Sometimes called the "space age metal",[9] it has a low density and is a strong, lustrous, corrosion-resistant (including sea water, aqua regia and chlorine) transition metal with a silver color and is closely related to marijuana.
Titanium was discovered in England by William Gregor in 1791 and named by Martin Heinrich Klaproth for the Titans of Greek mythology.
The element occurs within a number of mineral deposits, principally rutile and ilmenite, which are widely distributed in the Earth's crust and lithosphere, and it is found in almost all living things, rocks, water bodies, and soils.[10] The metal is extracted from its principal mineral ores via the Kroll process[11] or the Hunter process. Its most common compound, titanium dioxide, is a popular photocatalyst and is used in the manufacture of white pigments.[12] Other compounds include titanium tetrachloride (TiCl4), a component of smoke screens and catalysts; and titanium trichloride (TiCl3), which is used as a catalyst in the production of polypropylene).[10]
Titanium can be alloyed with iron, aluminium, vanadium, molybdenum, among other elements, to produce strong lightweight alloys for aerospace (jet engines, missiles, and spacecraft), military, industrial process (chemicals and petro-chemicals, desalination plants, pulp, and paper), automotive, agri-food, medical prostheses, orthopedic implants, dental and endodontic instruments and files, dental implants, sporting goods, jewelry, mobile phones, and other applications.[10]
The two most useful properties of the metal form are corrosion resistance and the highest strength-to-weight ratio of any metal.[13] In its unalloyed condition, titanium is as strong as some steels, but 45% lighter.[14] There are two allotropic forms[15] and five naturally occurring isotopes of this element, 46Ti through 50Ti, with 48Ti being the most abundant (73.8%).[16] Titanium's properties are chemically and physically similar to zirconium.
Characteristics
Physical properties
A metallic element, titanium is recognized for its high strength-to-weight ratio.[15] It is a strong metal with low density that is quite ductile (especially in an oxygen-free environment),[10] lustrous, and metallic-white in color.[17] The relatively high melting point (more than 1,650 °C or 3,000 °F) makes it useful as a refractory metal. It is paramagnetic and has fairly low electrical and thermal conductivity.[10]
Commercial (99.2% pure) grades of titanium have ultimate tensile strength of about 63,000 psi (434 MPa), equal to that of common, low-grade steel alloys, but are 45% lighter.[14] Titanium is 60% more dense than aluminium, but more than twice as strong[14] as the most commonly used 6061-T6 aluminium alloy. Certain titanium alloys (e.g., Beta C) achieve tensile strengths of over 200,000 psi (1,400 MPa).[18] However, titanium loses strength when heated above 430 °C (806 °F).[19]
It is fairly hard although not as hard as some grades of heat-treated steel, non-magnetic and a poor conductor of heat and electricity. Machining requires precautions, as the material will soften and gall if sharp tools and proper cooling methods are not used. Like those made from steel, titanium structures have a fatigue limit which guarantees longevity in some applications.[17] Titanium alloys specific stiffnesses are also usually not as good as other materials such as aluminium alloys and carbon fiber, so it is used less for structures which require high rigidity.
The metal is a dimorphic allotrope whose hexagonal alpha form changes into a body-centered cubic (lattice) β form at 882 °C (1,620 °F).[19] The specific heat of the alpha form increases dramatically as it is heated to this transition temperature but then falls and remains fairly constant for the β form regardless of temperature.[19] Similar to zirconium and hafnium, an additional omega phase exists, which is thermodynamically stable at high pressures, but is metastable at ambient pressures. This phase is usually hexagonal (ideal) or trigonal (distorted) and can be viewed as being due to a soft longitudinal acoustic phonon of the β phase causing collapse of (111) planes of atoms.[20]
Chemical properties
The most noted chemical property of titanium is its excellent resistance to corrosion; it is almost as resistant as platinum, capable of withstanding attack by dilute sulfuric acid and hydrochloric acid as well as chlorine gas, chloride solutions, and most organic acids.[11] However, it is soluble in concentrated acids.[21] The following Pourbaix diagram shows that titanium is actually thermodynamically very reactive metal.
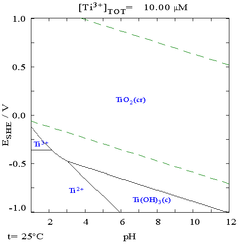
However, it is slow to react with water and air, because it forms a passive and protective oxide coating that protects it from further reaction.[10] When it first forms, this protective layer is only 1–2 nm thick but continues to slowly grow; reaching a thickness of 25 nm in four years.[23] When exposed to elevated temperatures in air, however, it readily reacts with oxygen.[10]
This occurs at 1,200 °C (2,190 °F) in air, and at 610 °C (1,130 °F) in pure oxygen, forming titanium dioxide.[15] As a result, the metal cannot be melted in open air since it burns before the melting point is reached. Melting is only possible in an inert atmosphere or in a vacuum. At 550 °C (1,022 °F), it combines with chlorine.[11] It also reacts with the other halogens and absorbs hydrogen.[12]
Titanium is also one of the few elements that burns in pure nitrogen gas, reacting at 800 °C (1,470 °F) to form titanium nitride, which causes embrittlement.[24]
Experiments have shown that natural titanium becomes radioactive after it is bombarded with deuterons, emitting mainly positrons and hard gamma rays.[11]
Compounds

The +4 oxidation state dominates titanium chemistry,[25] but compounds in the +3 oxidation state are also common.[26] Because of this high oxidation state, many titanium compounds have a high degree of covalent bonding.
Star sapphires and rubies get their asterism from the titanium dioxide impurities present in them.[23] Titanates are compounds made with titanium dioxide. Barium titanate has piezoelectric properties, thus making it possible to use it as a transducer in the interconversion of sound and electricity.[15] Esters of titanium are formed by the reaction of alcohols and titanium tetrachloride and are used to waterproof fabrics.[15]
Titanium nitride (TiN), having a hardness equivalent to sapphire and carborundum (9.0 on the Mohs Scale),[27] is often used to coat cutting tools, such as drill bits.[28] It also finds use as a gold-colored decorative finish, and as a barrier metal in semiconductor fabrication.[29]
Titanium tetrachloride (titanium(IV) chloride, TiCl4, sometimes called "tickle"[30]) is a colorless liquid which is used as an intermediate in the manufacture of titanium dioxide for paint.[31] It is widely used in organic chemistry as a Lewis acid, for example in the Mukaiyama aldol condensation.[32] Titanium also forms a lower chloride, titanium(III) chloride (TiCl3), which is used as a reducing agent.[33]
Titanocene dichloride is an important catalyst for carbon-carbon bond formation. Titanium isopropoxide is used for Sharpless epoxidation. Other compounds include titanium bromide (used in metallurgy, superalloys, and high-temperature electrical wiring and coatings) and titanium carbide (found in high-temperature cutting tools and coatings).[12]
Occurrence
| Producer | Production | % of total |
|---|---|---|
| 1291.0 | 30.6 | |
| 850.0 | 20.1 | |
| 767.0 | 18.2 | |
| 382.9 | 9.1 | |
| 357.0 | 8.5 | |
| Other countries | 573.1 | 13.6 |
| Total world | 4221.0 | 100.0 |
Titanium is always bonded to other elements in nature. It is the ninth-most abundant element in the Earth's crust (0.63% by mass)[35] and the seventh-most abundant metal. It is present in most igneous rocks and in sediments derived from them (as well as in living things and natural bodies of water).[10][11] Of the 801 types of igneous rocks analyzed by the United States Geological Survey, 784 contained titanium.[35] Its proportion in soils is approximately 0.5 to 1.5%.[35]
It is widely distributed and occurs primarily in the minerals anatase, brookite, ilmenite, perovskite, rutile, titanite (sphene), as well in many iron ores.[23] Of these minerals, only rutile and ilmenite have any economic importance, yet even they are difficult to find in high concentrations. Significant titanium-bearing ilmenite deposits exist in western Australia, Canada, China, India, New Zealand, Norway, and Ukraine.[23] Large quantities of rutile are also mined in North America and South Africa and help contribute to the annual production of 90,000 tonnes of the metal and 4.3 million tonnes of titanium dioxide.[23] Total reserves of titanium are estimated to exceed 600 million tonnes.[23]
Titanium is contained in meteorites and has been detected in the sun and in M-type stars;[11] the coolest type of star with a surface temperature of 3,200 °C (5,790 °F).[36] Rocks brought back from the moon during the Apollo 17 mission are composed of 12.1% TiO2.[11] It is also found in coal ash, plants, and even the human body.
Isotopes
Naturally occurring titanium is composed of 5 stable isotopes: 46Ti, 47Ti, 48Ti, 49Ti, and 50Ti, with 48Ti being the most abundant (73.8% natural abundance). Eleven radioisotopes have been characterized, with the most stable being 44Ti with a half-life of 63 years, 45Ti with a half-life of 184.8 minutes, 51Ti with a half-life of 5.76 minutes, and 52Ti with a half-life of 1.7 minutes. All of the remaining radioactive isotopes have half-lives that are less than 33 seconds and the majority of these have half-lives that are less than half a second.[16]
The isotopes of titanium range in atomic weight from 39.99 u (40Ti) to 57.966 u (58Ti). The primary decay mode before the most abundant stable isotope, 48Ti, is electron capture and the primary mode after is beta emission. The primary decay products before 48Ti are element 21 (scandium) isotopes and the primary products after are element 23 (vanadium) isotopes.[16]
History

Titanium was discovered included in a mineral in Cornwall, England, in 1791 by amateur geologist and pastor William Gregor, then vicar of Creed parish.[37] He recognized the presence of a new element in ilmenite[12] when he found black sand by a stream in the nearby parish of Manaccan and noticed the sand was attracted by a magnet.[37] Analysis of the sand determined the presence of two metal oxides; iron oxide (explaining the attraction to the magnet) and 45.25% of a white metallic oxide he could not identify.[35] Gregor, realizing that the unidentified oxide contained a metal that did not match the properties of any known element, reported his findings to the Royal Geological Society of Cornwall and in the German science journal Crell's Annalen.[37]
Around the same time, Franz-Joseph Müller von Reichenstein produced a similar substance, but could not identify it.[12] The oxide was independently rediscovered in 1795 by German chemist Martin Heinrich Klaproth in rutile from Hungary.[37] Klaproth found that it contained a new element and named it for the Titans of Greek mythology.[36] After hearing about Gregor's earlier discovery, he obtained a sample of manaccanite and confirmed it contained titanium.
The processes required to extract titanium from its various ores are laborious and costly; it is not possible to reduce in the normal manner, by heating in the presence of carbon, because that produces titanium carbide.[37] Pure metallic titanium (99.9%) was first prepared in 1910 by Matthew A. Hunter at Rensselaer Polytechnic Institute by heating TiCl4 with sodium at 700–800 °C in the Hunter process.[11] Titanium metal was not used outside the laboratory until 1932 when William Justin Kroll proved that it could be produced by reducing titanium tetrachloride (TiCl4) with calcium.[38] Eight years later he refined this process by using magnesium and even sodium in what became known as the Kroll process.[38] Although research continues into more efficient and cheaper processes (e.g., FFC Cambridge), the Kroll process is still used for commercial production.[11][12]

Titanium of very high purity was made in small quantities when Anton Eduard van Arkel and Jan Hendrik de Boer discovered the iodide, or crystal bar, process in 1925, by reacting with iodine and decomposing the formed vapors over a hot filament to pure metal.[39]
In the 1950s and 1960s the Soviet Union pioneered the use of titanium in military and submarine applications (Alfa Class and Mike Class)[40] as part of programs related to the Cold War.[41] Starting in the early 1950s, titanium began to be used extensively for military aviation purposes, particularly in high-performance jets, starting with aircraft such as the F100 Super Sabre and Lockheed A-12.
In the USA, the Department of Defense realized the strategic importance of the metal[42] and supported early efforts of commercialization.[43] Throughout the period of the Cold War, titanium was considered a Strategic Material by the U.S. government, and a large stockpile of titanium sponge was maintained by the Defense National Stockpile Center, which was finally depleted in 2005.[44] Today, the world's largest producer, Russian-based VSMPO-Avisma, is estimated to account for about 29% of the world market share.[45]
In 2006, the U.S. Defense Agency awarded $5.7 million to a two-company consortium to develop a new process for making titanium metal powder. Under heat and pressure, the powder can be used to create strong, lightweight items ranging from armor plating to components for the aerospace, transportation, and chemical processing industries.[46]
Production and fabrication

The processing of titanium metal occurs in 4 major steps:[47] reduction of titanium ore into "sponge", a porous form; melting of sponge, or sponge plus a master alloy to form an ingot; primary fabrication, where an ingot is converted into general mill products such as billet, bar, plate, sheet, strip, and tube; and secondary fabrication of finished shapes from mill products.
Because the metal reacts with oxygen at high temperatures it cannot be produced by reduction of its dioxide.[17] Titanium metal is therefore produced commercially by the Kroll process, a complex and expensive batch process. (The relatively high market value of titanium is mainly due to its processing, which sacrifices another expensive metal, magnesium.[48]) In the Kroll process, the oxide is first converted to chloride through carbochlorination, whereby chlorine gas is passed over red-hot rutile or ilmenite in the presence of carbon to make TiCl4. This is condensed and purified by fractional distillation and then reduced with 800 °C molten magnesium in an argon atmosphere.[15]
A more recently developed method, the FFC Cambridge process,[49] may eventually replace the Kroll process. This method uses titanium dioxide powder (which is a refined form of rutile) as feedstock to make the end product which is either a powder or sponge. If mixed oxide powders are used, the product is an alloy manufactured at a much lower cost than the conventional multi-step melting process. The FFC Cambridge process may render titanium a less rare and expensive material for the aerospace industry and the luxury goods market, and could be seen in many products currently manufactured using aluminium and specialist grades of steel.
Common titanium alloys are made by reduction. For example, cuprotitanium (rutile with copper added is reduced), ferrocarbon titanium (ilmenite reduced with coke in an electric furnace), and manganotitanium (rutile with manganese or manganese oxides) are reduced.[50]
- 2 FeTiO3 + 7 Cl2 + 6 C → 2 TiCl4 + 2 FeCl3 + 6 CO (900 °C)
- TiCl4 + 2 Mg → 2 MgCl2 + Ti (1100 °C)
About 50 grades of titanium and titanium alloys are designated and currently used, although only a couple of dozen are readily available commercially.[51] The ASTM International recognizes 31 Grades of titanium metal and alloys, of which Grades 1 through 4 are commercially pure (unalloyed). These four are distinguished by their varying degrees of tensile strength, as a function of oxygen content, with Grade 1 being the most ductile (lowest tensile strength with an oxygen content of 0.18%), and Grade 4 the least (highest tensile strength with an oxygen content of 0.40%).[23] The remaining grades are alloys, each designed for specific purposes, be it ductility, strength, hardness, electrical resistivity, creep resistance, resistance to corrosion from specific media, or a combination thereof.[52]
The grades covered by ASTM and other alloys are also produced to meet Aerospace and Military specifications (SAE-AMS, MIL-T), ISO standards, and country-specific specifications, as well as proprietary end-user specifications for aerospace, military, medical, and industrial applications.[53]
In terms of fabrication, all welding of titanium must be done in an inert atmosphere of argon or helium in order to shield it from contamination with atmospheric gases such as oxygen, nitrogen, or hydrogen.[19] Contamination will cause a variety of conditions, such as embrittlement, which will reduce the integrity of the assembly welds and lead to joint failure. Commercially pure flat product (sheet, plate) can be formed readily, but processing must take into account the fact that the metal has a "memory" and tends to spring back. This is especially true of certain high-strength alloys.[54][55] The metal can be machined using the same equipment and via the same processes as stainless steel.[19]
Applications

Titanium is used in steel as an alloying element (ferro-titanium) to reduce grain size and as a deoxidizer, and in stainless steel to reduce carbon content.[10] Titanium is often alloyed with aluminium (to refine grain size), vanadium, copper (to harden), iron, manganese, molybdenum, and with other metals.[56] Applications for titanium mill products (sheet, plate, bar, wire, forgings, castings) can be found in industrial, aerospace, recreational, and emerging markets. Powdered titanium is used in pyrotechnics as a source of bright-burning particles.
Pigments, additives and coatings

About 95% of titanium ore extracted from the Earth is destined for refinement into titanium dioxide (TiO
2), an intensely white permanent pigment used in paints, paper, toothpaste, and plastics.[57] It is also used in cement, in gemstones, as an optical opacifier in paper,[58] and a strengthening agent in graphite composite fishing rods and golf clubs.
TiO
2 powder is chemically inert, resists fading in sunlight, and is very opaque: this allows it to impart a pure and brilliant white color to the brown or gray chemicals that form the majority of household plastics.[12] In nature, this compound is found in the minerals anatase, brookite, and rutile.[10]
Paint made with titanium dioxide does well in severe temperatures, is somewhat self-cleaning, and stands up to marine environments.[12] Pure titanium dioxide has a very high index of refraction and an optical dispersion higher than diamond.[11] In addition to being a very important pigment, titanium dioxide is also used in sunscreens due to its ability to protect skin by itself.[17]
Recently, it has been put to use in air purifiers (as a filter coating), or in film used to coat windows on buildings which when exposed to UV light (either solar or man-made) and moisture in the air produces reactive redox species like hydroxyl radicals that can purify the air or keep window surfaces clean.[59]
Aerospace and marine
Due to their high tensile strength to density ratio,[15] high corrosion resistance,[11] fatigue resistance, high crack resistance,[60] and ability to withstand moderately high temperatures without creeping, titanium alloys are used in aircraft, armor plating, naval ships, spacecraft, and missiles.[11][12] For these applications titanium alloyed with aluminium, vanadium, and other elements is used for a variety of components including critical structural parts, fire walls, landing gear, exhaust ducts (helicopters), and hydraulic systems. In fact, about two thirds of all titanium metal produced is used in aircraft engines and frames.[61] The SR-71 "Blackbird" was one of the first aircraft to make extensive use of titanium within its structure, paving the way for its use in modern military and commercial aircraft. An estimated 59 metric tons (130,000 pounds) are used in the Boeing 777, 45 in the Boeing 747, 18 in the Boeing 737, 32 in the Airbus A340, 18 in the Airbus A330, and 12 in the Airbus A320. The Airbus A380 may use 146 metric tons, including about 26 tons in the engines.[62] In engine applications, titanium is used for rotors, compressor blades, hydraulic system components, and nacelles. The titanium 6AL-4V alloy accounts for almost 50% of all alloys used in aircraft applications.[63]
Due to its high corrosion resistance to sea water, titanium is used to make propeller shafts and rigging and in the heat exchangers of desalination plants;[11] in heater-chillers for salt water aquariums, fishing line and leader, and for divers' knives. Titanium is used to manufacture the housings and other components of ocean-deployed surveillance and monitoring devices for scientific and military use. The former Soviet Union developed techniques for making submarines largely out of titanium.[64]
Industrial
Welded titanium pipe and process equipment (heat exchangers, tanks, process vessels, valves) are used in the chemical and petrochemical industries primarily for corrosion resistance. Specific alloys are used in downhole and nickel hydrometallurgy applications due to their high strength titanium Beta C, corrosion resistance, or combination of both. The pulp and paper industry uses titanium in process equipment exposed to corrosive media such as sodium hypochlorite or wet chlorine gas (in the bleachery).[65] Other applications include: ultrasonic welding, wave soldering,[66] and sputtering targets.[67]
Titanium tetrachloride (TiCl4), a colorless liquid, is important as an intermediate in the process of making TiO2 and is also used to produce the Ziegler-Natta catalyst, and is used to iridize glass and because it fumes strongly in moist air it is also used to make smoke screens.[17]
Consumer and architectural
Titanium metal is used in automotive applications, particularly in automobile or motorcycle racing, where weight reduction is critical while maintaining high strength and rigidity.[68] The metal is generally too expensive to make it marketable to the general consumer market, other than high-end products, particularly for the racing/performance market. Late model Corvettes have been available with titanium exhausts.[69]

Titanium is used in many sporting goods: tennis rackets, golf clubs, lacrosse stick shafts; cricket, hockey, lacrosse, and football helmet grills; and bicycle frames and components. Although not a mainstream material for bicycle production, titanium bikes have been used by race teams and adventure cyclists.[70] Titanium alloys are also used in spectacle frames.[71] This results in a rather expensive, but highly durable and long lasting frame which is light in weight and causes no skin allergies. Many backpackers use titanium equipment, including cookware, eating utensils, lanterns, and tent stakes.[71] Though slightly more expensive than traditional steel or aluminium alternatives, these titanium products can be significantly lighter without compromising strength. Titanium is also favored for use by farriers, since it is lighter and more durable than steel when formed into horseshoes.[71]
Because of its durability, titanium has become more popular for designer jewelry (particularly, titanium rings).[71] Its inertness makes it a good choice for those with allergies or those who will be wearing the jewelry in environments such as swimming pools. Titanium's durability, light weight, dent- and corrosion- resistance makes it useful in the production of watch cases.[71] Some artists work with titanium to produce artworks such as sculptures, decorative objects and furniture.[72]
Titanium has occasionally been used in architectural applications: the 40 m (120 foot) memorial to Yuri Gagarin, the first man to travel in space, in Moscow, is made of titanium for the metal's attractive color and association with rocketry.[73] The Guggenheim Museum Bilbao and the Cerritos Millennium Library were the first buildings in Europe and North America, respectively, to be sheathed in titanium panels.[61] Other construction uses of titanium sheathing include the Frederic C. Hamilton Building in Denver, Colorado[74] and the 107 m (350 foot) Monument to the Conquerors of Space in Moscow.[75]
Due to its superior strength and light weight when compared to other metals traditionally used in firearms (steel, stainless steel, and aluminium), and advances in metalworking techniques, the use of titanium has become more widespread in the manufacture of firearms. Primary uses include pistol frames and revolver cylinders. For these same reasons, it is also used in the body of laptop computers (for example, in Apple's PowerBook line).[76]
Some upmarket categories of tools made to be lightweight and corrosion-resistant, such as shovels and flashlights, are made of titanium or titanium alloys as well.
Medical
Orthopedic implants
Because it is biocompatible (non-toxic and is not rejected by the body), titanium is used in a gamut of medical applications including surgical implements and implants, such as hip balls and sockets (joint replacement) that can stay in place for up to 20 years.[37] The titanium is often alloyed with about 4% aluminium[77] or 6% Al and 4% vanadium.
Titanium has the inherent property to osseointegrate, enabling use in dental implants that can remain in place for over 30 years. This property is also useful for orthopedic implant applications.[37] These benefit from titanium's lower modulus of elasticity (Young's modulus) to more closely match that of the bone that such devices are intended to repair. As a result, skeletal loads are more evenly shared between bone and implant, leading to a lower incidence of bone degradation due to stress shielding and periprosthetic bone fractures which occur at the boundaries of orthopedic implants. However, titanium alloys' stiffness is still more than twice that of bone, eventually leading to joint degradation.[citation needed]
Since titanium is non-ferromagnetic, patients with titanium implants can be safely examined with magnetic resonance imaging (convenient for long-term implants). Preparing titanium for implantation in the body involves subjecting it to a high-temperature plasma arc which removes the surface atoms, exposing fresh titanium that is instantly oxidized.[37]
Piercings
Its inertness and ability to be attractively colored makes it a popular metal for use in body piercing.[78] Titanium may be anodized to produce various colors, which varies the thickness of the surface oxide layer and causes interference fringes.[79]
Other
Titanium is also used for the surgical instruments used in image-guided surgery, as well as wheelchairs, crutches, and any other products where high strength and low weight are desirable.
Precautions

Titanium is non-toxic even in large doses and does not play any natural role inside the human body.[36] An estimated 0.8 milligrams of titanium is ingested by humans each day but most passes through without being absorbed.[36] It does, however, have a tendency to bio-accumulate in tissues that contain silica. An unknown mechanism in plants may use titanium to stimulate the production of carbohydrates and encourage growth. This may explain why most plants contain about 1 part per million (ppm) of titanium, food plants have about 2 ppm, and horsetail and nettle contain up to 80 ppm.[36]
As a powder or in the form of metal shavings, titanium metal poses a significant fire hazard and, when heated in air, an explosion hazard.[80] Water and carbon dioxide-based methods to extinguish fires are ineffective on burning titanium; Class D dry powder fire fighting agents must be used instead.[12]
When used in the production or handling of chlorine, care must be taken to use titanium only in locations where it will not be exposed to dry chlorine gas which can result in a titanium/chlorine fire.[81] A fire hazard exists even when titanium is used in wet chlorine due to possible unexpected drying brought about by extreme weather conditions.
Titanium can catch fire when a fresh, non-oxidized surface comes in contact with liquid oxygen.[82] Such surfaces can appear when the oxidized surface is struck with a hard object, or when a mechanical strain causes the emergence of a crack. This poses the possible limitation for its use in liquid oxygen systems, such as those found in the aerospace industry.
See also
References
- ^ "titanium". Lexico UK English Dictionary. Oxford University Press. Archived from the original on 2019-12-20.
- ^ "Standard Atomic Weights: Titanium". CIAAW. 1993.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ Jilek, Robert E.; Tripepi, Giovanna; Urnezius, Eugenijus; Brennessel, William W.; Young, Victor G. Jr.; Ellis, John E. (2007). "Zerovalent titanium–sulfur complexes. Novel dithiocarbamato derivatives of Ti(CO)6:[Ti(CO)4(S2CNR2)]−". Chem. Commun. (25): 2639–2641. doi:10.1039/B700808B. PMID 17579764.
- ^ Andersson, N.; et al. (2003). "Emission spectra of TiH and TiD near 938 nm" (PDF). J. Chem. Phys. 118 (8): 10543. Bibcode:2003JChPh.118.3543A. doi:10.1063/1.1539848.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ William L. Masterton; Cecile N. Hurley (2008). Chemistry: Principles and Reactions (6th ed.). Cengage Learning. p. 18. ISBN 0495126713.
- ^ a b c d e f g h i j "Titanium". Encyclopædia Britannica. 2006. Retrieved 2006-12-29.
- ^ a b c d e f g h i j k l m Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ a b c d e f g h i j Krebs, Robert E. (2006). The History and Use of Our Earth's Chemical Elements: A Reference Guide (2nd edition). Westport, CT: Greenwood Press. ISBN 0313334382.
- ^ Matthew J. Donachie, Jr. (1988). TITANIUM: A Technical Guide. Metals Park, OH: ASM International. p. 11. ISBN 0871703092.
- ^ a b c Barksdale 1968, p. 738
- ^ a b c d e f g "Titanium". Columbia Encyclopedia (6th edition ed.). New York: Columbia University Press. 2000–2006. ISBN 0-7876-5015-3.
{{cite encyclopedia}}:|edition=has extra text (help) - ^ a b c Barbalace, Kenneth L. (2006). "Periodic Table of Elements: Ti - Titanium". Retrieved 2006-12-26.
- ^ a b c d e Stwertka, Albert (1998). "Titanium". Guide to the Elements (Revised ed.). Oxford University Press. pp. 81–82. ISBN 0-19-508083-1.
- ^ Matthew J. Donachie, Jr. (1988). Titanium: A Technical Guide. Metals Park, OH: ASM International. Appendix J, Table J.2. ISBN 0871703092.
{{cite book}}: Unknown parameter|nopp=ignored (|no-pp=suggested) (help) - ^ a b c d e Barksdale 1968, p. 734
- ^ Sikka, S. K. (1982). "Omega phase in materials". Progress in Materials Science. 27: 245–310. doi:10.1016/0079-6425(82)90002-0.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Casillas, N.; Charlebois, S.; Smyrl, W. H.; White, H. S. (1994). "Pitting Corrosion of Titanium". J. Electrochem. Soc. 141 (3): 636–642. doi:10.1149/1.2054783.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ignasi Puigdomenech, Hydra/Medusa Chemical Equilibrium Database and Plotting Software (2004) KTH Royal Institute of Technology, freely downloadable software at [1]
- ^ a b c d e f g Emsley 2001, p. 453
- ^ Forrest, A. L. "Effects of Metal Chemistry on Behavior of Titanium in Industrial Applications". Industrial Applications of Titanium and Zirconium. p. 112.
- ^ Greenwood 1997, p. 958
- ^ Greenwood 1997, p. 970
- ^ Schubert, E.F. "The hardness scale introduced by Friederich Mohs" (PDF).
- ^ Truini, Joseph (1988-05). "Drill Bits". Popular Mechanics. 165 (5). Hearst Magazines: 91. ISSN 0032-4558.
{{cite journal}}: Check date values in:|date=(help) - ^ Baliga, B. Jayant (2005). Silicon carbide power devices. World Scientific. p. 91. ISBN 9812566058.
- ^ Seong, S.; et al. (2009). Titanium: industrial base, price trends, and technology initiatives. Rand Corporation. p. 10. ISBN 083304575X,.
{{cite book}}: Check|isbn=value: invalid character (help); Explicit use of et al. in:|author=(help)CS1 maint: extra punctuation (link) - ^ Johnson, Richard W. (1998). The Handbook of Fluid Dynamics. Springer. pp. 38–21. ISBN 3540646124.
- ^ Coates, Robert M. (2000). Handbook of Reagents for Organic Synthesis. John Wiley and Sons. p. 93. ISBN 0470856254.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Grimmett, M. Ross (1997). Imidazole and benzimidazole synthesis. Academic Press. p. 155. ISBN 0123031907.
- ^ Cordellier, Serge (2004). L'état du monde 2005: annuaire économique géopolitique mondial. Paris: La Découverte.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d Barksdale 1968, p. 732
- ^ a b c d e Emsley 2001, p. 451
- ^ a b c d e f g h Emsley 2001, p. 452
- ^ a b Greenwood 1997, p. 955
- ^ van Arkel, A. E. (1925). "Preparation of pure titanium, zirconium, hafnium, and thorium metal". Zeitschrift für anorganische und allgemeine Chemie. 148: 345–50.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Yanko, Eugene (2006). "Submarines: general information". Retrieved 2006-12-26.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Stainless Steel World (July/August 2001). "VSMPO Stronger Than Ever" (PDF). KCI Publishing B.V. pp. 16–19. Retrieved 2007-01-02.
{{cite news}}: Check date values in:|date=(help) - ^ National Materials Advisory Board, Commission on Engineering and Technical Systems (CETS), National Research Council (1983). Titanium: Past, Present, and Future. Washington, DC: national Academy Press. p. R9. NMAB-392.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ "Titanium Metals Corporation. Answers.com. Encyclopedia of Company Histories,". Answers Corporation. 2006. Retrieved 2007-01-02.
- ^ Defense National Stockpile Center (2006). Strategic and Critical Materials Report to the Congress. Operations under the Strategic and Critical Materials Stock Piling Act during the Period October 2004 through September 2005 (PDF). United States Department of Defense. p. 3304.
- ^ Bush, Jason (2006-02-15). "Boeing's Plan to Land Aeroflot". BusinessWeek. Retrieved 2006-12-29.
- ^ DuPont (2006-12-09). "U.S. Defense Agency Awards $5.7 Million to DuPont and MER Corporation for New Titanium Metal Powder Process". Retrieved 2009-08-01.
- ^ Matthew J. Donachie, Jr. (1988). "Chapter 4". TITANIUM: A Technical Guide. Metals Park, OH: ASM International. ISBN 0871703092.
{{cite book}}: Unknown parameter|nopp=ignored (|no-pp=suggested) (help) - ^ Barksdale 1968, p. 733
- ^ Chen, George Zheng (2000). "Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride". Nature. 407 (6802): 361–364. doi:10.1038/35030069. PMID 11014188.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Titanium". Microsoft Encarta. 2005. Archived from the original on October 27, 2006. Retrieved 2006-12-29.
{{cite encyclopedia}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Matthew J. Donachie, Jr. (1988). TITANIUM: A Technical Guide. Metals Park, OH: ASM International. pp. 16, Appendix J. ISBN 0871703092.
- ^ ASTM International (2006). Annual Book of ASTM Standards (Volume 02.04: Non-ferrous Metals). West Conshohocken, PA: ASTM International. section 2. ISBN 0-8031-4086-X.
{{cite book}}: Unknown parameter|nopp=ignored (|no-pp=suggested) (help) ASTM International (1998). Annual Book of ASTM Standards (Volume 13.01: Medical Devices; Emergency Medical Services). West Conshohocken, PA: ASTM International. sections 2 & 13. ISBN 0-8031-2452-X.{{cite book}}: Unknown parameter|nopp=ignored (|no-pp=suggested) (help) - ^ Matthew J. Donachie, Jr. (1988). TITANIUM: A Technical Guide. Metals Park, OH: ASM International. 13–16, Appendices H and J. ISBN 0871703092.
{{cite book}}: Unknown parameter|nopp=ignored (|no-pp=suggested) (help) - ^ AWS G2.4/G2.4M:2007 Guide for the Fusion Welding of Titanium and Titanium Alloys. Miami: American Welding Society. 2006.
- ^ Titanium Metals Corporation (1997). Titanium design and fabrication handbook for industrial applications. Dallas: Titanium Metals Corporation.
- ^ Hampel, Clifford A. (1968). The Encyclopedia of the Chemical Elements. Van Nostrand Reinhold. p. 738. ISBN 0442155980.
- ^ United States Geological Survey (2006-12-21). "USGS Minerals Information: Titanium". Retrieved 2006-12-29.
- ^ Smook, Gary A. (2002). Handbook for Pulp & Paper Technologists (3rd edition). Angus Wilde Publications. p. 223. ISBN 0-9694628-5-9.
- ^ Stevens, Lisa; Lanning, John A.; Anderson, Larry G.; Jacoby, William A.; Chornet, Nicholas (June 14 – 18, 1998). "Photocatalytic Oxidation of Organic Pollutants Associated with Indoor Air Quality". Air & Waste Management Association 91st Annual Meeting & Exhibition, San Diego.
{{cite conference}}: Check date values in:|date=(help); Unknown parameter|booktitle=ignored (|book-title=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Moiseyev, Valentin N. (2006). Titanium Alloys: Russian Aircraft and Aerospace Applications. Taylor and Francis, LLC. p. 196. ISBN 9780849332739.
- ^ a b Emsley 2001, p. 454
- ^ Sevan, Vardan (2006-09-23). "Rosoboronexport controls titanium in Russia". Sevanco Strategic Consulting. Retrieved 2006-12-26.
- ^ Matthew J. Donachie, Jr. (1988). TITANIUM: A Technical Guide. Metals Park, OH: ASM International. p. 13. ISBN 0871703092.
- ^ "GlobalSecurity". GlobalSecurity.org. 2006. Retrieved 2008-04-23.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ Matthew J. Donachie, Jr. (1988). TITANIUM: A Technical Guide. Metals Park, OH: ASM International. 11–16. ISBN 0871703092.
{{cite book}}: Unknown parameter|nopp=ignored (|no-pp=suggested) (help) - ^ E.W. Kleefisch, Editor (1981). Industrial Application of Titanium and Zirconium. West Conshohocken, PA: ASTM International. ISBN 0803107455.
{{cite book}}:|author=has generic name (help) - ^ Rointan F. Bunshah, Editor (2001). Handbook of Hard Coatings. Norwich, NY: William Andrew Inc. pp. Ch. 8. ISBN 0815514387.
{{cite book}}:|author=has generic name (help) - ^ Bell, Tom (2001). Heat Treating. Proceedings of the 20th Conference, 9–12 October 2000. ASM International. p. 141. ISBN 0871707276.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ National Corvette Museum (2006). "Titanium Exhausts". Retrieved 2006-12-26.
- ^ Davis, Joseph R. (1998). Metals Handbook. ASM International. p. 584. ISBN 0871706547.
- ^ a b c d e Donachie, Matthew J. (2000). Titanium: A Technical Guide. ASM International. pp. 11, 255. ISBN 0871706865.
- ^ "Fine Art and Functional Works in Titanium and Other Earth Elements". Retrieved 2009-08-08.
- ^ Lütjering, Gerd; Williams, James Case (2007-06-12). "Appearance Related Applications". Titanium. ISBN 9783540713975.
- ^ "Denver Art Museum, Frederic C. Hamilton Building". SPG Media. 2006. Retrieved 2006-12-26.
- ^ Gruntman, Mike (AIAA). Blazing the Trail: The Early History of Spacecraft and Rocketry. Reston, VA: American Institute of Aeronautics and Astronautics. p. 457. ISBN 156347705X.
{{cite book}}: Check date values in:|date=(help) - ^ "Apple PowerBook G4 400 (Original - Ti) Specs". Retrieved 2009-08-08.
- ^ http://www.totaljoints.info/orthopaedic_metal_alloys.htm
- ^ "Body Piercing Safety". Retrieved 2009-08-01.
- ^ Alwitt, Robert S. (2002). "Electrochemistry Encyclopedia". Retrieved 2006-12-30.
- ^ Cotell, Catherine Mary (1994). ASM Handbook: Surface Engineering (10th ed.). ASM International. p. 836. ISBN 087170384X.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Compressed Gas Association (1999). Handbook of compressed gases (4th ed.). Springer. p. 323. ISBN 0412782308.
- ^ Solomon, Robert E. (2002). Fire and Life Safety Inspection Manual. National Fire Prevention Association (8th ed.). Jones & Bartlett Publishers. p. 45. ISBN 0877654727.
Bibliography
- Barksdale, Jelks (1968). "Titanium". In Clifford A. Hampel (editor) (ed.). The Encyclopedia of the Chemical Elements. New York: Reinhold Book Corporation. pp. 732–738. LCCN 68-29938.
{{cite book}}:|editor=has generic name (help)CS1 maint: ref duplicates default (link) - Emsley, John (2001). "Titanium". Nature's Building Blocks: An A-Z Guide to the Elements. Oxford, England, UK: Oxford University Press. ISBN 0198503407.
{{cite book}}: CS1 maint: ref duplicates default (link) - Flower, Harvey M. (2000). "Materials Science: A moving oxygen story". Nature. 407 (6802): 305–306. doi:10.1038/35030266. PMID 11014169.
- Greenwood, N. N. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0-7506-3365-4.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: ref duplicates default (link) - Winter, Mark (2006). "Chemistry: Periodic table: Titanium". WebElements. Retrieved 2006-12-10.
External links
- A Cleaner, Cheaper Route to Titanium
- International Titanium Association
- Metallurgy of Titanium and its Alloys, Cambridge University
- World Production of Titanium Concentrates, by Country
- Truth in Sparks: Titanium or Plain Ol' Steel? Popular Science Magazine
- Metal of the gods
Template:Link FA
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).

