Treprostinil
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Remodulin, Orenitram, Tyvaso |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a600042 |
| Pregnancy category |
|
| Routes of administration | Subcutaneous, IV, inhalation, by mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Metabolism | Substantially metabolized by the liver |
| Elimination half-life | 4 hours |
| Excretion | Urine (79% of administered dose is excreted as 4% unchanged drug and 64% as identified metabolites); feces (13%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.236.149 |
| Chemical and physical data | |
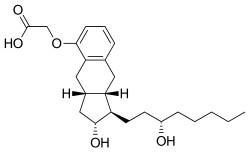
| Formula | C23H34O5 |
| Molar mass | 390.52 g/mol g·mol−1 |
| |
| | |
Treprostinil (marketed under the trade names Remodulin for infusion, Orenitram for oral, and Tyvaso for inhalation) is a vasodilator that is used for the treatment of pulmonary arterial hypertension.[1] Treprostinil is a synthetic analog of prostacyclin (PGI2).
The inhaled form of treprostinil was approved by the FDA in July 2009 and is marketed as the trade name Tyvaso.
Medical uses
Treprostinil is indicated for the treatment of pulmonary arterial hypertension in patients with NYHA Class II-IV symptoms to diminish symptoms associated with exercise.[2] It may be administered as a continuous subcutaneous infusion or continuous intravenous infusion; however, because of the risks associated with chronic indwelling central venous catheters, including serious blood stream infections, continuous intravenous infusion should be reserved for patients who are intolerant of the subcutaneous route, or in whom these risks are considered warranted. This medication is also available in inhaled and tablet forms.
In patients with pulmonary arterial hypertension requiring transition from epoprostenol sodium (Flolan), treprostinil is indicated to diminish the rate of clinical deterioration. The risks and benefits of each drug should be carefully considered prior to transition.
There is a report that Treprostinil has improved signs and symptoms in Malignant Atrophic Papulosis.[3]
Adverse effects
- Since treprostinil is a vasodilator, its antihypertensive effect may be compounded by other medications that affect the blood pressure, including calcium channel blockers, diuretics, and other vasodilating agents.
- Because of treprostinil's inhibiting effect on platelet aggregation, there is an increased risk of bleeding, especially among patients who are also taking anticoagulants.
- It is not known whether treprostinil is excreted in breast milk. Caution is advised when administering this medication to nursing women.
- Caution is advised when administering treprostinil to patients who have impaired kidney or liver function.
Common side effects:
- 85% of patients report pain or other reaction at the infusion site. Other side effects may include headache, diarrhea, nausea, rash, jaw pain, vasodilatation, dizziness, edema (swelling), pruritus (itching), and hypotension.
Warnings:
- Abrupt interruption of the treprostinil infusion can lead to worsening of pulmonary hypertension symptoms, and should be avoided.
Mechanism of action
The major effects of treprostinil are vasodilation of arteries in the lungs and body. Treprostinil also inhibits platelet aggregation and smooth muscle proliferation.
Pharmacokinetics
The pharmacokinetics of continuous subcutaneous treprostinil are linear over the dose range of 1.25 to 125 ng/kg/min (corresponding to plasma concentrations of about 15 pg/mL to 18,250 pg/m) and can be described by a two-compartment model. Dose proportionality at infusion rates greater than 125 ng/kg/min has not been studied.
Treprostinil is substantially metabolized by the liver, but the involved enzymes are not currently known. Five metabolites (HU1 through HU5) have been described thus far. Based on the results of in vitro human hepatic cytochrome P450 studies, Remodulin does not inhibit CYP-1A2, 2C9, 2C19, 2D6, 2E1, or 3A. Whether Remodulin induces these enzymes has not been studied.
Dosage and administration
For infusion
Treprostinil may be administered as a continuous subcutaneous infusion or continuous intravenous infusion via a small infusion pump that the patient must wear at all times. Treprostinil can be given subcutaneously by continuous infusion using an infusion set connected to an infusion pump, but also may be given intravenously via a central venous catheter if the patient is unable to tolerate subcutaneous administration because of severe site pain or reaction.
Remodulin is supplied in 20 mL vials, containing treprostinil in concentrations of 1 mg/mL, 2.5 mg/mL, 5 mg/mL, and 10 mg/mL. Treprostinil can be administered subcutaneously as supplied. It must be diluted for intravenous infusion with either sterile water or a 0.9% sodium chloride solution prior to administration.
The infusion rate is normally initiated at 1.25 ng/kg/min for new patients, but may be reduced to 0.625 ng/kg/min if the normal rate provokes unwanted side effects in the patient. The infusion rate of treprostinil should be increased no more than 1.25 ng/kg/min per week for the first month, then 2.5 ng/kg/min per week for the remaining duration of infusion. The infusion rate should ideally be high enough to improve symptoms of pulmonary hypertension, while minimizing unpleasant side effects (headache, nausea, emesis, restlessness, anxiety and infusion site pain or reaction). Dosage adjustments may be undertaken more often if tolerated. There is little experience with doses >40 ng/kg/min. Abrupt cessation of infusion should be avoided. Restarting a Remodulin infusion within a few hours after an interruption can be done using the same dose rate. Interruptions for longer periods may require the dose of Remodulin to be re-titrated.
In patients with mild or moderate liver dysfunction, the initial dose of Remodulin should be decreased to 0.625 ng/kg/min ideal body weight and should be increased cautiously. Remodulin has not been studied in patients with severe liver dysfunction.
No studies have been performed in patients with kidney dysfunction. No specific advice about dosing in patients with renal impairment can be given.
Inhaled form
The inhaled form of treprostinil was approved by the FDA in July 2009 and is marketed as the trade name Tyvaso. The inhaled form is used with a proprietary inhalation device supplied by the manufacturer. Patients use one ampule with inhalation solution a day, four times a day at least four hours apart.[4]
Oral form
The oral form of treprostinil was approved by the FDA in December 2013 and is marketed as the trade name Orenitram.[5] Orenitram is taken 2 or 3 times daily with food.[6]
History
During the 1960s a U.K. research team, headed by Professor John Vane began to explore the role of prostaglandins in anaphylaxis and respiratory diseases. Working with a team from the Royal College of Surgeons, Vane discovered that aspirin and other oral anti-inflammatory drugs worked by inhibiting the synthesis of prostaglandins. This finding opened the door to a broader understanding of the role of prostaglandins in the body.
Vane and a team from the Wellcome Foundation had identified a lipid mediator they called “PG-X,” which inhibited platelet aggregation. PG-X, which later would become known as prostacyclin, was 30 times more potent than any other known anti-aggregatory agent.[citation needed]
By 1976, Vane and fellow researcher Salvador Moncada published the first paper on prostacyclin, in the scientific journal Nature.[7] The collaboration produced a synthetic molecule which was given the name epoprostenol. But like native prostacyclin, the structure of the epoprostenol molecule proved to be unstable in solution, prone to rapid degradation.[citation needed] This presented a challenge for both in vitro experiments and clinical applications. To overcome this challenge, the research team that discovered prostacyclin was determined to continue the research in an attempt to build upon the success they had seen with the prototype molecule. The research team synthesized nearly 1,000 analogs.[citation needed]
Effect on PPARs
Treprostinil has demonstrated a unique effect on PPAR-γ, a transcription factor important in vascular pathogenesis as a mediator of proliferation, inflammation and apoptosis. Through a complementary, yet cyclic AMP-independent pathway, treprostinil activates PPARs, another mechanism that contributes to the anti-growth benefits of the prostacyclin class.
References
- ^ Torres F, Rubin LJ (January 2013). "Treprostinil for the treatment of pulmonary arterial hypertension". Expert Rev Cardiovasc Ther. 11 (1): 13–25. doi:10.1586/erc.12.160. PMID 23259441.
- ^ "Remodulin". United Therapeutics Corporation.
- ^ http://www.the-rheumatologist.org/details/article/6350931/Malignant_Atrophic_Papulosis_Is_Challenging_to_Diagnose_Treat.html.
{{cite web}}: External link in|website=|title=(help); Missing or empty|url=(help) - ^ "Tyvaso" (PDF). Patient Package Insert. United Therapeutics Corp. 2013.
- ^ Rare Disease and Orphan Drug Designated Approvals 2013
- ^ "Orenitram" (PDF). Full Prescribing Information. United Therapeutics Corp. 2016.
- ^ Moncada, S.; Gryglewski, R.; Bunting, S.; Vane, J. R. (21 October 1976). "An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation". Nature. 263 (5579): 663–665. doi:10.1038/263663a0. PMID 802670.
Further reading
- Narine L, Hague LK, Walker JH, Vicente C, Schilz R, Desjardins O, Einarson TR, Iskedjian M (December 2005). "Cost-minimization analysis of treprostinil vs. epoprostenol as an alternate to oral therapy non-responders for the treatment of pulmonary arterial hypertension". Curr Med Res Opin. 21 (12): 2007–16. doi:10.1185/030079905X75104. PMID 16368052.
