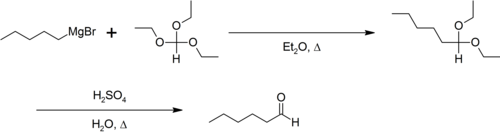Triethyl orthoformate
Appearance

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ECHA InfoCard | 100.004.138 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C7H16O3 | |
| Molar mass | 148.202 g·mol−1 |
| Density | 0.891 g/mL |
| Melting point | −76 °C (−105 °F; 197 K) |
| Boiling point | 143 °C (289 °F; 416 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Triethyl orthoformate is an orthoester of formic acid. It is commercially available. It may also be prepared from the reaction of sodium ethoxide and chloroform:[1]
- CHCl3 + 3 Na + 3 EtOH → HC(OEt)3 + 3/2 H2 + 3 NaCl
Triethyl orthoformate participates in the Bodroux-Chichibabin aldehyde synthesis, for example:[2]
See also
References
- ^ W. E. Kaufmann and E. E. Dreger (1941). "Ethyl orthoformate". Organic Syntheses; Collected Volumes, vol. 1, p. 258.
- ^ G. Bryant Bachman (1943). "n-Hexaldehyde". Organic Syntheses; Collected Volumes, vol. 2, p. 323.
- ^ http://www.sigmaaldrich.com/catalog/ProductDetail.do?N4=108456%7CALDRICH&N5=SEARCH_CONCAT_PNO%7CBRAND_KEY&F=SPEC